Structure and function of the Rad9-binding region of the DNA-damage checkpoint adaptor TopBP1
- PMID: 20724438
- PMCID: PMC3017600
- DOI: 10.1093/nar/gkq743
Structure and function of the Rad9-binding region of the DNA-damage checkpoint adaptor TopBP1
Erratum in
- Nucleic Acids Res. 2013 Apr;41(8):4741
Abstract
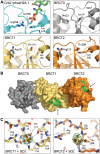
TopBP1 is a scaffold protein that coordinates activation of the DNA-damage-checkpoint response by coupling binding of the 9-1-1 checkpoint clamp at sites of ssDNA, to activation of the ATR-ATRIP checkpoint kinase complex. We have now determined the crystal structure of the N-terminal region of human TopBP1, revealing an unexpected triple-BRCT domain structure. The arrangement of the BRCT domains differs significantly from previously described tandem BRCT domain structures, and presents two distinct sites for binding phosphopeptides in the second and third BRCT domains. We show that the site in the second but not third BRCT domain in the N-terminus of TopBP1, provides specific interaction with a phosphorylated motif at pSer387 in Rad9, which can be generated by CK2.
Figures




References
-
- Smits VA, Warmerdam DO, Martin Y, Freire R. Mechanisms of ATR-mediated checkpoint signalling. Front. Biosci. 2010;15:840–853. - PubMed
-
- Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. - PubMed
-
- Majka J, Burgers PM. The PCNA-RFC families of DNA clamps and clamp loaders. Prog. Nucleic Acid Res. Mol. Biol. 2004;78:227–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

