Characterization of an interplay between a Mycobacterium tuberculosis MazF homolog, Rv1495 and its sole DNA topoisomerase I
- PMID: 20724443
- PMCID: PMC3001090
- DOI: 10.1093/nar/gkq737
Characterization of an interplay between a Mycobacterium tuberculosis MazF homolog, Rv1495 and its sole DNA topoisomerase I
Erratum in
-
Correction to 'Characterization of an interplay between a Mycobacterium tuberculosis MazF homolog, Rv1495 and its sole DNA topoisomerase I'.Nucleic Acids Res. 2025 Jan 7;53(1):gkae1226. doi: 10.1093/nar/gkae1226. Nucleic Acids Res. 2025. PMID: 39656664 Free PMC article. No abstract available.
Abstract
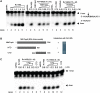
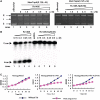
The MazEF systems are thought to contribute to the capacity for long-term dormancy observed in the human pathogen, Mycobacterium tuberculosis. However, except for their functions as mRNA interferases, little is known regarding any additional cellular functions of these systems in the pathogen. In the present study, we observed a negative interplay between MazF protein Rv1495 and the sole M. tuberculosis DNA topoisomerase I (MtbTopA) with respect to protein functions. Through its C-terminal domain, MtbTopA physically interacted with and inhibited the mRNA cleavage activity of Rv1495. Rv1495, in turn, inhibited the DNA cleavage activity of MtbTopA as well as its function of relaxation of supercoiled DNA. An N-terminus fragment of Rv1495, designated Rv1495-N(29-56), lost mRNA cleavage activity, but retained a significant physical interaction and inhibitory effect on TopA proteins from both M. tuberculosis and M. smegmatis. This fragment, although less effective than the full-length protein, was able to inhibit mycobacterial growth when expressed through a recombinant plasmid in M. smegmatis. The Rv1495 physically interacted with the M. smegmatis TopA both in vitro and in vivo. Our findings imply that MazEF systems can affect bacterial survival by a novel mechanism that allows direct modulation of M. tuberculosis topoisomerase I.
Figures





Similar articles
-
The mRNA interferases, MazF-mt3 and MazF-mt7 from Mycobacterium tuberculosis target unique pentad sequences in single-stranded RNA.Mol Microbiol. 2008 Aug;69(3):559-69. doi: 10.1111/j.1365-2958.2008.06284.x. Epub 2008 Jun 28. Mol Microbiol. 2008. PMID: 18485066 Free PMC article.
-
Physical and functional interaction between D-ribokinase and topoisomerase I has opposite effects on their respective activity in Mycobacterium smegmatis and Mycobacterium tuberculosis.Arch Biochem Biophys. 2011 Aug 15;512(2):135-42. doi: 10.1016/j.abb.2011.05.018. Epub 2011 Jun 12. Arch Biochem Biophys. 2011. PMID: 21683681
-
Inhibition of Mycobacterium tuberculosis topoisomerase I by m-AMSA, a eukaryotic type II topoisomerase poison.Biochem Biophys Res Commun. 2014 Apr 18;446(4):916-20. doi: 10.1016/j.bbrc.2014.03.029. Epub 2014 Mar 15. Biochem Biophys Res Commun. 2014. PMID: 24642256
-
DNA topoisomerase I and DNA gyrase as targets for TB therapy.Drug Discov Today. 2017 Mar;22(3):510-518. doi: 10.1016/j.drudis.2016.11.006. Epub 2016 Nov 14. Drug Discov Today. 2017. PMID: 27856347 Review.
-
The discovery of mRNA interferases: implication in bacterial physiology and application to biotechnology.J Cell Physiol. 2006 Dec;209(3):670-6. doi: 10.1002/jcp.20801. J Cell Physiol. 2006. PMID: 17001682 Review.
Cited by
-
Regulated Expression Systems for Mycobacteria and Their Applications.Microbiol Spectr. 2014;2(1):03. doi: 10.1128/microbiolspec.MGM2-0018-2013. Microbiol Spectr. 2014. PMID: 25485177 Free PMC article.
-
Emerging Roles of Toxin-Antitoxin Modules in Bacterial Pathogenesis.Molecules. 2016 Jun 17;21(6):790. doi: 10.3390/molecules21060790. Molecules. 2016. PMID: 27322231 Free PMC article. Review.
-
A TetR family transcriptional factor directly regulates the expression of a 3-methyladenine DNA glycosylase and physically interacts with the enzyme to stimulate its base excision activity in Mycobacterium bovis BCG.J Biol Chem. 2014 Mar 28;289(13):9065-75. doi: 10.1074/jbc.M113.528919. Epub 2014 Feb 7. J Biol Chem. 2014. PMID: 24509852 Free PMC article.
-
Toxin-Antitoxin Systems in Clinical Pathogens.Toxins (Basel). 2016 Jul 20;8(7):227. doi: 10.3390/toxins8070227. Toxins (Basel). 2016. PMID: 27447671 Free PMC article. Review.
-
Induced ectopic expression of HigB toxin in Mycobacterium tuberculosis results in growth inhibition, reduced abundance of a subset of mRNAs and cleavage of tmRNA.Mol Microbiol. 2013 Oct;90(1):195-207. doi: 10.1111/mmi.12358. Epub 2013 Aug 23. Mol Microbiol. 2013. PMID: 23927792 Free PMC article.
References
-
- Zhu L, Zhang Y, Teh JS, Zhang J, Connell N, Rubin H, Inouye M. Characterization of mRNA interferases from Mycobacterium tuberculosis. J. Biol. Chem. 2006;281:18638–18643. - PubMed
-
- Engelberg-Kulka H, Hazan R, Amitai S. mazEF: a chromosomal toxin-antitoxin module that triggers programmed cell death in bacteria. J. Cell Sci. 2005;118:4327–4332. - PubMed
-
- Gerdes K, Christensen SK, Løbner-Olesen A. Prokaryotic toxin-antitoxin stress response loci. Nat. Rev. Microbiol. 2005;3:371–382. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

