2E8 binds to the high affinity I-domain in a metal ion-dependent manner: a second generation monoclonal antibody selectively targeting activated LFA-1
- PMID: 20724473
- PMCID: PMC2963386
- DOI: 10.1074/jbc.M110.111591
2E8 binds to the high affinity I-domain in a metal ion-dependent manner: a second generation monoclonal antibody selectively targeting activated LFA-1
Abstract
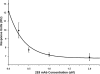
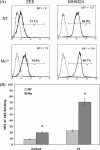
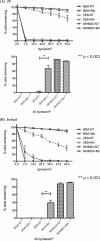
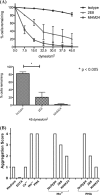
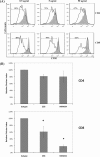
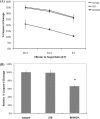
The activation of leukocyte function-associated antigen-1 (LFA-1) plays a critical role in regulating immune responses. The metal ion-dependent adhesion site on the I-domain of LFA-1 α(L) subunit is the key recognition site for ligand binding. Upon activation, conformation changes in the I-domain can lead LFA-1 from the low affinity state to the high affinity (HA) state. Using the purified HA I-domain locked by disulfide bonds for immunization, we developed an mAb, 2E8, that specifically binds to cells expressing the HA LFA-1. The surface plasmon resonance analysis has shown that 2E8 only binds to the HA I-domain and that the dissociation constant (K(D)) for HA I-domain is 197 nm. The binding of 2E8 to the HA I-domain is metal ion-dependent, and the affinity decreased as Mn(2+) was replaced sequentially by Mg(2+) and Ca(2+). Surface plasmon resonance analysis demonstrates that 2E8 inhibits the interaction of HA I-domain and ICAM-1. Furthermore, we found that 2E8 can detect activated LFA-1 on both JY and Jurkat cells using flow cytometry and parallel plate adhesion assay. In addition, 2E8 inhibits JY cell adhesion to human umbilical vein endothelial cells and homotypic aggregation. 2E8 treatment reduces the proliferation of both human CD4(+) and CD8(+) T cells upon OKT3 stimulation without the impairment of their cytolytic function. Taken together, these data demonstrate that 2E8 is specific for the high affinity form of LFA-1 and that 2E8 inhibits LFA-1/ICAM-1 interactions. As a novel activation-specific monoclonal antibody, 2E8 is a potentially useful reagent for blocking high affinity LFA-1 and modulating T cell activation in research and therapeutics.
Figures


References
-
- Springer T. A. (1994) Cell. 76, 301–314 - PubMed
-
- Dustin M. L. (2003) Ann. N.Y. Acad. Sci. 987, 51–59 - PubMed
-
- Carman C. V., Springer T. A. (2003) Curr. Opin. Cell Biol. 15, 547–556 - PubMed
-
- Hogg N., Smith A., McDowall A., Giles K., Stanley P., Laschinger M., Henderson R. (2004) Immunol. Lett. 92, 51–54 - PubMed
-
- Hynes R. O. (2003) Science. 300, 755–756 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

