Carbon monoxide promotes VEGF expression by increasing HIF-1alpha protein level via two distinct mechanisms, translational activation and stabilization of HIF-1alpha protein
- PMID: 20724477
- PMCID: PMC2952213
- DOI: 10.1074/jbc.M110.131284
Carbon monoxide promotes VEGF expression by increasing HIF-1alpha protein level via two distinct mechanisms, translational activation and stabilization of HIF-1alpha protein
Abstract
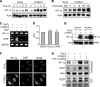
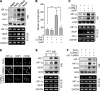
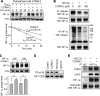
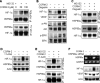
Carbon monoxide (CO) plays a significant role in vascular functions. We here examined the molecular mechanism by which CO regulates HIF-1 (hypoxia-inducible transcription factor-1)-dependent expression of vascular endothelial growth factor (VEGF), which is an important angiogenic factor. We found that astrocytes stimulated with CORM-2 (CO-releasing molecule) promoted angiogenesis by increasing VEGF expression and secretion. CORM-2 also induced HO-1 (hemeoxygenase-1) expression and increased nuclear HIF-1α protein level, without altering its promoter activity and mRNA level. VEGF expression was inhibited by treatment with HIF-1α siRNA and a hemeoxygenase inhibitor, indicating that CO stimulates VEGF expression via up-regulation of HIF-1α protein level, which is partially associated with HO-1 induction. CORM-2 activated the translational regulatory proteins p70(S6k) and eIF-4E as well as phosphorylating their upstream signal mediators Akt and ERK. These translational signal events and HIF-1α protein level were suppressed by inhibitors of phosphatidylinositol 3-kinase (PI3K), MEK, and mTOR, suggesting that the PI3K/Akt/mTOR and MEK/ERK pathways are involved in a translational increase in HIF-1α. In addition, CORM-2 also increased stability of the HIF-1α protein by suppressing its ubiquitination, without altering the proline hydroxylase-dependent HIF-1α degradation pathway. CORM-2 increased HIF-1α/HSP90α interaction, which is responsible for HIF-1α stabilization, and HSP90-specific inhibitors decreased this interaction, HIF-1α protein level, and VEGF expression. Furthermore, HSP90α knockdown suppressed CORM-2-induced increases in HIF-1α and VEGF protein levels. These results suggest that CO stimulates VEGF production by increasing HIF-1α protein level via two distinct mechanisms, translational stimulation and protein stabilization of HIF-1α.
Figures

Similar articles
-
Carbon Monoxide Potentiation of L-Type Ca2+ Channel Activity Increases HIF-1α-Independent VEGF Expression via an AMPKα/SIRT1-Mediated PGC-1α/ERRα Axis.Antioxid Redox Signal. 2017 Jul 1;27(1):21-36. doi: 10.1089/ars.2016.6684. Epub 2016 Sep 28. Antioxid Redox Signal. 2017. PMID: 27554679
-
Isoflurane preconditioning increases survival of rat skin random-pattern flaps by induction of HIF-1α expression.Cell Physiol Biochem. 2013;31(4-5):579-91. doi: 10.1159/000350078. Epub 2013 Apr 26. Cell Physiol Biochem. 2013. PMID: 23635649
-
Ginsenoside-Rg1 mediates a hypoxia-independent upregulation of hypoxia-inducible factor-1α to promote angiogenesis.Angiogenesis. 2011 Dec;14(4):515-22. doi: 10.1007/s10456-011-9235-z. Epub 2011 Oct 2. Angiogenesis. 2011. PMID: 21964931 Free PMC article.
-
HIF-1α pathway: role, regulation and intervention for cancer therapy.Acta Pharm Sin B. 2015 Sep;5(5):378-89. doi: 10.1016/j.apsb.2015.05.007. Epub 2015 Jun 6. Acta Pharm Sin B. 2015. PMID: 26579469 Free PMC article. Review.
-
Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis.Acta Biochim Pol. 2003;50(1):49-59. Acta Biochim Pol. 2003. PMID: 12673346 Review.
Cited by
-
Extra Virgin Olive Oil Modulates Vasodilator Enzyme Level by Repairing Angiogenesis Function in Rat Model of Preeclampsia.J Family Reprod Health. 2020 Mar;14(1):38-44. J Family Reprod Health. 2020. PMID: 32863837 Free PMC article.
-
Metabolic Regulation of Hypoxia-Inducible Factors in Hypothalamus.Front Endocrinol (Lausanne). 2021 Mar 8;12:650284. doi: 10.3389/fendo.2021.650284. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33763034 Free PMC article. Review.
-
USP33 deubiquitinates and stabilizes HIF-2alpha to promote hypoxia response in glioma stem cells.EMBO J. 2022 Apr 4;41(7):e109187. doi: 10.15252/embj.2021109187. Epub 2022 Feb 22. EMBO J. 2022. PMID: 35191554 Free PMC article.
-
Repair Mechanisms of the Neurovascular Unit after Ischemic Stroke with a Focus on VEGF.Int J Mol Sci. 2021 Aug 9;22(16):8543. doi: 10.3390/ijms22168543. Int J Mol Sci. 2021. PMID: 34445248 Free PMC article. Review.
-
Modulation of Nrf2/HO-1 by Natural Compounds in Lung Cancer.Antioxidants (Basel). 2023 Mar 16;12(3):735. doi: 10.3390/antiox12030735. Antioxidants (Basel). 2023. PMID: 36978983 Free PMC article. Review.
References
-
- Otterbein L. E., Zuckerbraun B. S., Haga M., Liu F., Song R., Usheva A., Stachulak C., Bodyak N., Smith R. N., Csizmadia E., Tyagi S., Akamatsu Y., Flavell R. J., Billiar T. R., Tzeng E., Bach F. H., Choi A. M., Soares M. P. (2003) Nat. Med. 9, 183–190 - PubMed
-
- Chauveau C., Bouchet D., Roussel J. C., Mathieu P., Braudeau C., Renaudin K., Tesson L., Soulillou J. P., Iyer S., Buelow R., Anegon I. (2002) Am. J. Transplant. 2, 581–592 - PubMed
-
- Foresti R., Bani-Hani M. G., Motterlini R. (2008) Intensive Care Med. 34, 649–658 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

