The residual nonadrenergic contractile response to nerve stimulation of the mouse prostate is mediated by acetylcholine but not ATP in a comparison with the mouse vas deferens
- PMID: 20724483
- PMCID: PMC2967401
- DOI: 10.1124/jpet.110.172130
The residual nonadrenergic contractile response to nerve stimulation of the mouse prostate is mediated by acetylcholine but not ATP in a comparison with the mouse vas deferens
Abstract
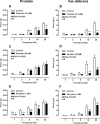
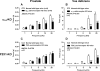
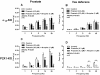
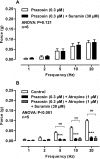
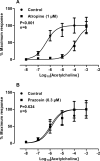
Neuronal release of noradrenaline is primarily responsible for the contraction of prostatic smooth muscle in all species, and this forms the basis for the use of α(1)-adrenoceptor antagonists as pharmacotherapies for benign prostatic hyperplasia. Previous studies in mice have demonstrated that a residual nonadrenergic component to nerve stimulation remains after α(1)-adrenoceptor antagonism. In the guinea pig and rat prostate and the vas deferens of guinea pigs, rats, and mice, ATP is the mediator of this residual contraction. This study investigates the mediator of residual contraction in the mouse prostate. Whole prostates from wild-type, α(1A)-adrenoceptor, and P2X1-purinoceptor knockout mice were mounted in organ baths, and the isometric force that tissues developed in response to electrical field stimulation or exogenously applied agonists was recorded. Deletion of the P2X1 purinoceptor did not affect nerve-mediated contraction. Furthermore, the P2-purinoceptor antagonist suramin (30 μM) failed to attenuate nerve-mediated contractions in wild-type, α(1A)-adrenoceptor, or P2X1-purinoceptor knockout mice. Atropine (1 μM) attenuated contraction in prostates taken from wild-type mice. In the presence of prazosin (0.3 μM) or guanethidine (10 μM), or in prostates taken from α(1A)-adrenoceptor knockout mice, residual nerve-mediated contraction was abolished by atropine (1 μM), but not suramin (30 μM). Exogenously administered acetylcholine elicited reproducible concentration-dependent contractions of the mouse prostate that were atropine-sensitive (1 μM), but not prazosin-sensitive (0.3 μM). Acetylcholine, but not ATP, mediates the nonadrenergic component of contraction in the mouse prostate. This cholinergic component of prostatic contraction is mediated by activation of muscarinic receptors.
Figures


Similar articles
-
Neural ATP release and its alpha 2-adrenoceptor-mediated modulation in guinea-pig vas deferens.Naunyn Schmiedebergs Arch Pharmacol. 1993 Oct;348(4):358-66. doi: 10.1007/BF00171334. Naunyn Schmiedebergs Arch Pharmacol. 1993. PMID: 7904050
-
Contractions of the mouse prostate elicited by acetylcholine are mediated by M(3) muscarinic receptors.J Pharmacol Exp Ther. 2011 Dec;339(3):870-7. doi: 10.1124/jpet.111.186841. Epub 2011 Sep 1. J Pharmacol Exp Ther. 2011. PMID: 21885618
-
The alpha1A-adrenoceptor gene is required for the alpha1L-adrenoceptor-mediated response in isolated preparations of the mouse prostate.Br J Pharmacol. 2008 Sep;155(1):103-9. doi: 10.1038/bjp.2008.245. Epub 2008 Jun 16. Br J Pharmacol. 2008. PMID: 18552869 Free PMC article.
-
Opposite modulation of noradrenaline and ATP release in guinea-pig vas deferens through prejunctional beta-adrenoceptors: evidence for the beta 2 subtype.Naunyn Schmiedebergs Arch Pharmacol. 1996 Apr;353(5):564-71. doi: 10.1007/BF00169177. Naunyn Schmiedebergs Arch Pharmacol. 1996. PMID: 8740151
-
Investigation of postjunctional alpha1- and alpha2-adrenoceptor subtypes in vas deferens from wild-type and alpha(2A/D)-adrenoceptor knockout mice.Br J Pharmacol. 2003 Mar;138(6):1069-76. doi: 10.1038/sj.bjp.0705137. Br J Pharmacol. 2003. PMID: 12684262 Free PMC article.
Cited by
-
Male contraception via simultaneous knockout of α1A-adrenoceptors and P2X1-purinoceptors in mice.Proc Natl Acad Sci U S A. 2013 Dec 17;110(51):20825-30. doi: 10.1073/pnas.1318624110. Epub 2013 Dec 2. Proc Natl Acad Sci U S A. 2013. PMID: 24297884 Free PMC article.
-
Age-related changes in the innervation of the prostate gland: implications for prostate cancer initiation and progression.Organogenesis. 2013 Jul-Sep;9(3):206-15. doi: 10.4161/org.24843. Epub 2013 May 14. Organogenesis. 2013. PMID: 23872639 Free PMC article. Review.
-
Ectonucleotidases and purinergic receptors in mouse prostate gland.Am J Clin Exp Urol. 2025 Apr 25;13(2):145-155. doi: 10.62347/NGQZ2940. eCollection 2025. Am J Clin Exp Urol. 2025. PMID: 40400995 Free PMC article.
-
Novel drug targets for the pharmacotherapy of benign prostatic hyperplasia (BPH).Br J Pharmacol. 2011 Jul;163(5):891-907. doi: 10.1111/j.1476-5381.2011.01332.x. Br J Pharmacol. 2011. PMID: 21410684 Free PMC article. Review.
-
Regulation of nerve-evoked contractions of rabbit vas deferens by acetylcholine.Physiol Rep. 2015 Sep;3(9):e12520. doi: 10.14814/phy2.12520. Physiol Rep. 2015. PMID: 26359240 Free PMC article.
References
-
- Arver S, Sjöstrand NO. ( 1982) Functions of adrenergic and cholinergic nerves in canine effectors of seminal emission. Acta Physiol Scand 115: 67– 77 - PubMed
-
- Banks FC, Knight GE, Calvert RC, Thompson CS, Morgan RJ, Burnstock G. ( 2006) The purinergic component of human vas deferens contraction. Fertil Steril 85: 932– 939 - PubMed
-
- Buljubasich R, Ventura S. ( 2004) Adenosine 5′-triphosphate and noradrenaline are excitatory cotransmitters to the fibromuscular stroma of the guinea pig prostate gland. Eur J Pharmacol 499: 335– 344 - PubMed
-
- Burnstock G, Verkhratsky A. ( 2010) Vas deferens–a model used to establish sympathetic cotransmission. Trends Pharmacol Sci 31: 131– 139 - PubMed
-
- Caine M, Raz S, Zeigler M. ( 1975) Adrenergic and cholinergic receptors in human prostate, prostatic capsule and bladder neck. Br J Urol 47: 193– 202 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

