The absence of LPA receptor 2 reduces the tumorigenesis by ApcMin mutation in the intestine
- PMID: 20724530
- PMCID: PMC2993170
- DOI: 10.1152/ajpgi.00321.2010
The absence of LPA receptor 2 reduces the tumorigenesis by ApcMin mutation in the intestine
Abstract
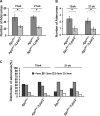
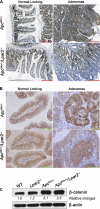
Lysophosphatidic acid (LPA) is a lipid mediator that mediates several effects that promote cancer progress. The LPA receptor type 2 (LPA(2)) expression is often elevated in several types of cancers, including colorectal cancer (CRC). In this study, we investigated the role of LPA(2) in the development of intestinal adenomas by comparing Apc(Min/+) mice with Apc(Min/+)/Lpar2(-/-) mice. There were 50% fewer intestinal adenomas in Apc(Min/+)/Lpar2(-/-) mice than Apc(Min/+) mice. Smaller-size adenomas (<1 mm) were found at higher frequencies in Apc(Min/+)/Lpar2(-/-) mice compared with Apc(Min/+) mice at the two age groups examined. The expression level of LPA(2) correlated with increased size of intestinal adenomas. Reduced tumor multiplicity and size in Apc(Min/+)/Lpar2(-/-) mice correlated with decreased proliferation of intestinal epithelial cells. Apc(Min/+)/Lpar2(-/-) mice showed an increased level of apoptosis, suggesting that LPA(2)-mediated signaling stimulates intestinal tumor development and progress by regulating both cell proliferation and survival. In addition, the expression levels of Krüpple-like factor 5 (KLF5), β-catenin, cyclin D1, c-Myc, and hypoxia-inducible factor-1α (HIF-1α) were significantly altered in Apc(Min/+)/Lpar2(-/-) mice compared with Apc(Min/+) mice. In vitro studies using HCT116 cells showed that LPA induced cyclin D1, c-Myc, and HIF-1α expression, which was attenuated by knockdown of LPA(2). In summary, intestinal tumor initiated by Apc mutations is altered by LPA(2)-mediated signaling, which regulates tumor growth and survival by altering multiple targets.
Figures





Similar articles
-
Intestinal epithelial cell-specific CD98 expression regulates tumorigenesis in Apc(Min/+) mice.Lab Invest. 2012 Aug;92(8):1203-12. doi: 10.1038/labinvest.2012.83. Epub 2012 May 28. Lab Invest. 2012. PMID: 22641098
-
Stromal Indian hedgehog signaling is required for intestinal adenoma formation in mice.Gastroenterology. 2015 Jan;148(1):170-180.e6. doi: 10.1053/j.gastro.2014.10.006. Epub 2014 Oct 13. Gastroenterology. 2015. PMID: 25307863
-
Haploinsufficiency of Krüppel-like factor 5 rescues the tumor-initiating effect of the Apc(Min) mutation in the intestine.Cancer Res. 2009 May 15;69(10):4125-33. doi: 10.1158/0008-5472.CAN-08-4402. Epub 2009 May 12. Cancer Res. 2009. PMID: 19435907 Free PMC article.
-
Mutated K-ras(Asp12) promotes tumourigenesis in Apc(Min) mice more in the large than the small intestines, with synergistic effects between K-ras and Wnt pathways.Int J Exp Pathol. 2009 Oct;90(5):558-74. doi: 10.1111/j.1365-2613.2009.00667.x. Int J Exp Pathol. 2009. PMID: 19765110 Free PMC article.
-
Berries as chemopreventive dietary constituents--a mechanistic approach with the ApcMin/+ mouse.Asia Pac J Clin Nutr. 2008;17 Suppl 1:123-5. Asia Pac J Clin Nutr. 2008. PMID: 18296318 Review.
Cited by
-
Lysophosphatidic acid type 2 receptor agonists in targeted drug development offer broad therapeutic potential.J Lipid Res. 2019 Mar;60(3):464-474. doi: 10.1194/jlr.S091744. Epub 2019 Jan 28. J Lipid Res. 2019. PMID: 30692142 Free PMC article. Review.
-
Higher LPA2 and LPA6 mRNA Levels in Hepatocellular Carcinoma Are Associated with Poorer Differentiation, Microvascular Invasion and Earlier Recurrence with Higher Serum Autotaxin Levels.PLoS One. 2016 Sep 1;11(9):e0161825. doi: 10.1371/journal.pone.0161825. eCollection 2016. PLoS One. 2016. PMID: 27583415 Free PMC article.
-
Identification of Differentially Methylated CpG Sites in Fibroblasts from Keloid Scars.Biomedicines. 2020 Jun 28;8(7):181. doi: 10.3390/biomedicines8070181. Biomedicines. 2020. PMID: 32605309 Free PMC article.
-
Lysophosphatidic Acid Receptor 1 Is Important for Intestinal Epithelial Barrier Function and Susceptibility to Colitis.Am J Pathol. 2018 Feb;188(2):353-366. doi: 10.1016/j.ajpath.2017.10.006. Epub 2017 Nov 9. Am J Pathol. 2018. PMID: 29128569 Free PMC article.
-
Inhibition of autotaxin alleviates inflammation and increases the expression of sodium-dependent glucose cotransporter 1 and Na+/H+ exchanger 3 in SAMP1/Fc mice.Am J Physiol Gastrointest Liver Physiol. 2018 Nov 1;315(5):G762-G771. doi: 10.1152/ajpgi.00215.2018. Epub 2018 Aug 17. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 30118349 Free PMC article.
References
-
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell 103: 311–320, 2000 - PubMed
-
- Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 50: 157–186, 2010 - PubMed
-
- Chun J. Lysophospholipid receptors: implications for neural signaling. Crit Rev Neurobiol 13: 151–168, 1999 - PubMed
-
- Contos JJA, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa2 (Edg4) and lpa1/lpa2 (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa2. Mol Cell Biol 22: 6921–6929, 2002 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

