Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery
- PMID: 20724594
- PMCID: PMC2947295
- DOI: 10.2353/ajpath.2010.091245
Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery
Abstract
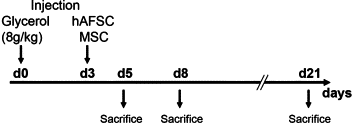
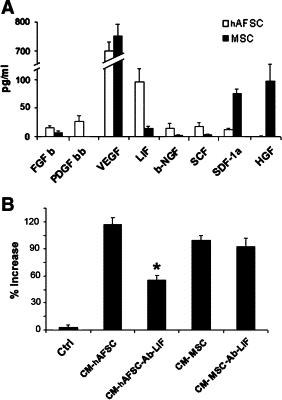
Stem cells isolated from human amniotic fluid are gaining attention with regard to their therapeutic potential. In this work, we investigated whether these cells contribute to tubular regeneration after experimental acute kidney injury. Cells expressing stem cell markers with multidifferentiative potential were isolated from human amniotic fluid. The regenerative potential of human amniotic fluid stem cells was compared with that of bone marrow-derived human mesenchymal stem cells. We found that the intravenous injection of 3.5 × 10(5) human amniotic fluid stem cells into nonimmune-competent mice with glycerol-induced acute kidney injury was followed by rapid normalization of renal function compared with injection of mesenchymal stem cells. Both stem cell types showed enhanced tubular cell proliferation and reduced apoptosis. Mesenchymal stem cells were more efficient in inducing proliferation than amniotic fluid-derived stem cells, which, in contrast, were more antiapoptotic. Both cell types were found to accumulate within the peritubular capillaries and the interstitium, but amniotic fluid stem cells were more persistent than mesenchymal stem cells. In vitro experiments demonstrated that the two cell types produced different cytokines and growth factors, suggesting that a combination of different mediators is involved in their biological actions. These results suggest that the amniotic fluid-derived stem cells may improve renal regeneration in acute kidney injury, but they are not more effective than mesenchymal stem cells.
Figures




Similar articles
-
Human amniotic fluid stem cell preconditioning improves their regenerative potential.Stem Cells Dev. 2012 Jul 20;21(11):1911-23. doi: 10.1089/scd.2011.0333. Epub 2011 Dec 23. Stem Cells Dev. 2012. PMID: 22066606 Free PMC article.
-
Osteoblastic differentiation potential of human amniotic fluid-derived mesenchymal stem cells in different culture conditions.Acta Histochem. 2018 Nov;120(8):701-712. doi: 10.1016/j.acthis.2018.07.006. Epub 2018 Aug 2. Acta Histochem. 2018. PMID: 30078494
-
The In Vitro Differentiation of GDNF Gene-Engineered Amniotic Fluid-Derived Stem Cells into Renal Tubular Epithelial-Like Cells.Stem Cells Dev. 2018 May 1;27(9):590-599. doi: 10.1089/scd.2017.0120. Epub 2018 Apr 19. Stem Cells Dev. 2018. PMID: 29649411
-
Stem cells from amniotic fluid--Potential for regenerative medicine.Best Pract Res Clin Obstet Gynaecol. 2016 Feb;31:45-57. doi: 10.1016/j.bpobgyn.2015.08.009. Epub 2015 Sep 10. Best Pract Res Clin Obstet Gynaecol. 2016. PMID: 26542929 Review.
-
Therapeutic Potential of Amniotic Fluid Derived Mesenchymal Stem Cells Based on their Differentiation Capacity and Immunomodulatory Properties.Curr Stem Cell Res Ther. 2019;14(4):327-336. doi: 10.2174/1574888X14666190222201749. Curr Stem Cell Res Ther. 2019. PMID: 30806325 Review.
Cited by
-
Fibroblasts as a practical alternative to mesenchymal stem cells.J Transl Med. 2018 Jul 27;16(1):212. doi: 10.1186/s12967-018-1536-1. J Transl Med. 2018. PMID: 30053821 Free PMC article. Review.
-
Intravenous renal cell transplantation with SAA1-positive cells prevents the progression of chronic renal failure in rats with ischemic-diabetic nephropathy.Am J Physiol Renal Physiol. 2013 Dec 15;305(12):F1804-12. doi: 10.1152/ajprenal.00097.2013. Epub 2013 Oct 16. Am J Physiol Renal Physiol. 2013. PMID: 24133118 Free PMC article.
-
In search of mechanisms associated with mesenchymal stem cell-based therapies for acute kidney injury.Clin Biochem Rev. 2013 Nov;34(3):131-44. Clin Biochem Rev. 2013. PMID: 24353358 Free PMC article. Review.
-
Amniotic Fluid Derived Stem Cells with a Renal Progenitor Phenotype Inhibit Interstitial Fibrosis in Renal Ischemia and Reperfusion Injury in Rats.PLoS One. 2015 Aug 21;10(8):e0136145. doi: 10.1371/journal.pone.0136145. eCollection 2015. PLoS One. 2015. PMID: 26295710 Free PMC article.
-
Amniotic fluid cells: current progress and emerging challenges in renal regeneration.Pediatr Nephrol. 2018 Jun;33(6):935-945. doi: 10.1007/s00467-017-3711-7. Epub 2017 Jun 15. Pediatr Nephrol. 2018. PMID: 28620747 Review.
References
-
- Little MH. Regrow or repair: potential regenerative therapies for the kidney. J Am Soc Nephrol. 2006;17:2390–2401. - PubMed
-
- Chhabra P, Brayman KL. The use of stem cells in kidney disease. Curr Opin Organ Transplant. 2009;14:72–78. - PubMed
-
- Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–325. - PubMed
-
- Humphreys BD, Duffield JS, Bonventre JV. Renal stem cells in recovery from acute kidney injury. Minerva Urol Nefrol. 2006;58:329–337. - PubMed
-
- Morigi M, Benigni A, Remuzzi G, Imberti B. The regenerative potential of stem cells in acute renal failure. Cell Transplant. 2006;15(Suppl 1):S111–S117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

