The calreticulin-binding sequence of thrombospondin 1 regulates collagen expression and organization during tissue remodeling
- PMID: 20724603
- PMCID: PMC2947268
- DOI: 10.2353/ajpath.2010.090903
The calreticulin-binding sequence of thrombospondin 1 regulates collagen expression and organization during tissue remodeling
Abstract
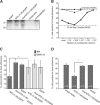
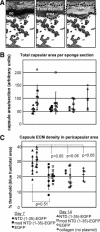
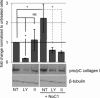
Amino acids 17-35 of the thrombospondin1 (TSP1) N-terminal domain (NTD) bind cell surface calreticulin to signal focal adhesion disassembly, cell migration, and anoikis resistance in vitro. However, the in vivo relevance of this signaling pathway has not been previously determined. We engineered local in vivo expression of the TSP1 calreticulin-binding sequence to determine the role of TSP1 in tissue remodeling. Surgical sponges impregnated with a plasmid encoding the secreted calreticulin-binding sequence [NTD (1-35)-EGFP] or a control sequence [mod NTD (1-35)-EGFP] tagged with enhanced green fluorescent protein were implanted subcutaneously in mice. Sponges expressing NTD (1-35)-EFGP formed a highly organized capsule despite no differences in cellular composition, suggesting stimulation of collagen deposition by the calreticulin-binding sequence of TSP1. TSP1, recombinant NTD, or a peptide of the TSP1 calreticulin-binding sequence (hep I) increased both collagen expression and matrix deposition by fibroblasts in vitro. TSP1 stimulation of collagen was inhibited by a peptide that blocks TSP1 binding to calreticulin, demonstrating the requirement for cell surface calreticulin. Collagen stimulation was independent of TGF-β activity and Smad phosphorylation but was blocked by an Akt inhibitor, suggesting that signaling through the Akt pathway is important for regulation of collagen through TSP1 binding to calreticulin. These studies identify a novel function for the NTD of TSP1 as a mediator of collagen expression and deposition during tissue remodeling.
Figures







Similar articles
-
Thrombospondin 1 binding to calreticulin-LRP1 signals resistance to anoikis.FASEB J. 2008 Nov;22(11):3968-79. doi: 10.1096/fj.07-104802. Epub 2008 Jul 24. FASEB J. 2008. PMID: 18653767 Free PMC article.
-
Thrombospondin signaling through the calreticulin/LDL receptor-related protein co-complex stimulates random and directed cell migration.J Cell Sci. 2003 Jul 15;116(Pt 14):2917-27. doi: 10.1242/jcs.00600. J Cell Sci. 2003. PMID: 12808019
-
Thrombospondin induces RhoA inactivation through FAK-dependent signaling to stimulate focal adhesion disassembly.J Biol Chem. 2004 Nov 19;279(47):48983-92. doi: 10.1074/jbc.M404881200. Epub 2004 Sep 13. J Biol Chem. 2004. PMID: 15371459
-
Thrombospondin1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms.Matrix Biol. 2012 Apr;31(3):178-86. doi: 10.1016/j.matbio.2012.01.006. Epub 2012 Jan 14. Matrix Biol. 2012. PMID: 22266026 Free PMC article. Review.
-
Thrombospondin 1 and Its Diverse Roles as a Regulator of Extracellular Matrix in Fibrotic Disease.J Histochem Cytochem. 2019 Sep;67(9):683-699. doi: 10.1369/0022155419851103. Epub 2019 May 22. J Histochem Cytochem. 2019. PMID: 31116066 Free PMC article. Review.
Cited by
-
Role of thrombospondin‑1 and thrombospondin‑2 in cardiovascular diseases (Review).Int J Mol Med. 2020 May;45(5):1275-1293. doi: 10.3892/ijmm.2020.4507. Epub 2020 Feb 20. Int J Mol Med. 2020. PMID: 32323748 Free PMC article. Review.
-
Cell-matrix interactions: focus on proteoglycan-proteinase interplay and pharmacological targeting in cancer.FEBS J. 2014 Nov;281(22):5023-42. doi: 10.1111/febs.12927. Epub 2014 Nov 6. FEBS J. 2014. PMID: 25333340 Free PMC article. Review.
-
Calreticulin regulates transforming growth factor-β-stimulated extracellular matrix production.J Biol Chem. 2013 May 17;288(20):14584-14598. doi: 10.1074/jbc.M112.447243. Epub 2013 Apr 5. J Biol Chem. 2013. PMID: 23564462 Free PMC article.
-
The role of the endoplasmic reticulum protein calreticulin in mediating TGF-β-stimulated extracellular matrix production in fibrotic disease.J Cell Commun Signal. 2018 Mar;12(1):289-299. doi: 10.1007/s12079-017-0426-2. Epub 2017 Oct 28. J Cell Commun Signal. 2018. PMID: 29080087 Free PMC article. Review.
-
Pericytes in hematogenous metastasis: mechanistic insights and therapeutic approaches.Cell Oncol (Dordr). 2025 Aug;48(4):921-941. doi: 10.1007/s13402-025-01073-6. Epub 2025 May 20. Cell Oncol (Dordr). 2025. PMID: 40392500 Free PMC article. Review.
References
-
- Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:1031–1037. - PubMed
-
- Alford AI, Hankenson KD. Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–757. - PubMed
-
- Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res. 2004;64:24–31. - PubMed
-
- McPherson J, Sage H, Bornstein P. Isolation and characterization of a glycoprotein secreted by aortic endothelial cells in culture. Apparent identity with platelet thrombospondin. J Biol Chem. 1981;256:11330–11336. - PubMed
-
- Raugi GJ, Mumby SM, Ready CA, Bornstein P. Location and partial characterization of the heparin-binding fragment of platelet thrombospondin. Thromb Res. 1984;36:165–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

