Mas-related G-protein-coupled receptors inhibit pathological pain in mice
- PMID: 20724664
- PMCID: PMC2936626
- DOI: 10.1073/pnas.1011221107
Mas-related G-protein-coupled receptors inhibit pathological pain in mice
Abstract
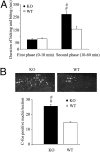
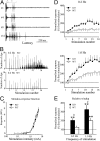
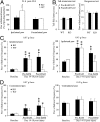
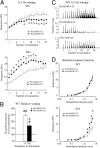
An important objective of pain research is to identify novel drug targets for the treatment of pathological persistent pain states, such as inflammatory and neuropathic pain. Mas-related G-protein-coupled receptors (Mrgprs) represent a large family of orphan receptors specifically expressed in small-diameter nociceptive primary sensory neurons. To determine the roles of Mrgprs in persistent pathological pain states, we exploited a mouse line in which a chromosomal locus spanning 12 Mrgpr genes was deleted (KO). Initial studies indicated that these KO mice show prolonged mechanical- and thermal-pain hypersensitivity after hind-paw inflammation compared with wild-type littermates. Here, we show that this mutation also enhances the windup response of dorsal-horn wide dynamic-range neurons, an electrophysiological model for the triggering of central pain sensitization. Deletion of the Mrgpr cluster also blocked the analgesic effect of intrathecally applied bovine adrenal medulla peptide 8-22 (BAM 8-22), an MrgprC11 agonist, on both inflammatory heat hyperalgesia and neuropathic mechanical allodynia. Spinal application of bovine adrenal medulla peptide 8-22 also significantly attenuated windup in wild-type mice, an effect eliminated in KO mice. These data suggest that members of the Mrgpr family, in particular MrgprC11, may constitute an endogenous inhibitory mechanism for regulating persistent pain in mice. Agonists for these receptors may, therefore, represent a class of antihyperalgesics for treating persistent pain with minimal side effects because of the highly specific expression of their targets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. - PubMed
-
- Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–1769. - PubMed
-
- Baron R. Mechanisms of disease: Neuropathic pain—a clinical perspective. Nat Clin Pract Neurol. 2006;2:95–106. - PubMed
-
- MacPherson RD. New directions in pain management. Drugs Today (Barc) 2002;38:135–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

