Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel
- PMID: 20724705
- PMCID: PMC2955818
- DOI: 10.1161/CIRCRESAHA.110.220673
Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel
Abstract
Rationale: Pyridine nucleotides regulate the cardiac Na(+) current (I(Na)) through generation of reactive oxygen species (ROS).
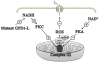
Objective: We investigated the source of ROS induced by elevated NADH.
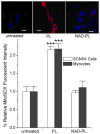
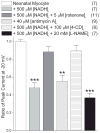
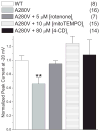
Methods and results: In human embryonic kidney (HEK) cells stably expressing the cardiac Na(+) channel, the decrease of I(Na) (52±9%; P<0.01) induced by cytosolic NADH application (100 μmol/L) was reversed by mitoTEMPO, rotenone, malonate, DIDS (4,4'-diisothiocyanatostilbene-2,2'-disulfonic acid), PK11195, and 4'-chlorodiazepam, a specific scavenger of mitochondrial superoxide and inhibitors of the mitochondrial complex I, complex II, voltage-dependent anion channels, and benzodiazepine receptor, respectively. Anti-mycin A (20 μmol/L), a complex III inhibitor known to generate ROS, decreased I(Na) (51±4%, P<0.01). This effect was blocked by NAD(+), forskolin, or rotenone. Inhibitors of complex IV, nitric oxide synthase, the NAD(P)H oxidases, xanthine oxidases, the mitochondrial permeability transition pore, and the mitochondrial ATP-sensitive K(+) channel did not change the NADH effect on I(Na). Analogous results were observed in cardiomyocytes. Rotenone, mitoTEMPO, and 4'-chlorodiazepam also blocked the mutant A280V GPD1-L (glycerol-3-phosphate dehydrogenase 1-like) effect on reducing I(Na), indicating a role for mitochondria in the Brugada syndrome caused by this mutation. Fluorescent microscopy confirmed mitochondrial ROS generation with elevated NADH and ROS inhibition by NAD(+).
Conclusions: Altering the oxidized to reduced NAD(H) balance can activate mitochondrial ROS production, leading to reduced I(Na). This signaling cascade may help explain the link between altered metabolism, conduction block, and arrhythmic risk.
Figures


Similar articles
-
Cardiac Na+ current regulation by pyridine nucleotides.Circ Res. 2009 Oct 9;105(8):737-45. doi: 10.1161/CIRCRESAHA.109.197277. Epub 2009 Sep 10. Circ Res. 2009. PMID: 19745168 Free PMC article.
-
Mitochondrial dysfunction causing cardiac sodium channel downregulation in cardiomyopathy.J Mol Cell Cardiol. 2013 Jan;54:25-34. doi: 10.1016/j.yjmcc.2012.10.011. Epub 2012 Nov 1. J Mol Cell Cardiol. 2013. PMID: 23123323 Free PMC article.
-
Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias.Circulation. 2007 Nov 13;116(20):2260-8. doi: 10.1161/CIRCULATIONAHA.107.703330. Epub 2007 Oct 29. Circulation. 2007. PMID: 17967977 Free PMC article.
-
Role of oxidants on calcium and sodium movement in healthy and diseased cardiac myocytes.Free Radic Biol Med. 2013 Oct;63:338-49. doi: 10.1016/j.freeradbiomed.2013.05.035. Epub 2013 Jun 1. Free Radic Biol Med. 2013. PMID: 23732518 Review.
-
Genetic Na+ channelopathies and sinus node dysfunction.Prog Biophys Mol Biol. 2008 Oct-Nov;98(2-3):171-8. doi: 10.1016/j.pbiomolbio.2008.10.003. Epub 2008 Nov 5. Prog Biophys Mol Biol. 2008. PMID: 19027778 Review.
Cited by
-
Ketamine induces toxicity in human neurons differentiated from embryonic stem cells via mitochondrial apoptosis pathway.Curr Drug Saf. 2012 Apr;7(2):106-19. doi: 10.2174/157488612802715663. Curr Drug Saf. 2012. PMID: 22873495 Free PMC article.
-
Post-translational modifications of the cardiac Na channel: contribution of CaMKII-dependent phosphorylation to acquired arrhythmias.Am J Physiol Heart Circ Physiol. 2013 Aug 15;305(4):H431-45. doi: 10.1152/ajpheart.00306.2013. Epub 2013 Jun 14. Am J Physiol Heart Circ Physiol. 2013. PMID: 23771687 Free PMC article. Review.
-
Redox control of cardiac excitability.Antioxid Redox Signal. 2013 Feb 1;18(4):432-68. doi: 10.1089/ars.2011.4234. Epub 2012 Aug 16. Antioxid Redox Signal. 2013. PMID: 22897788 Free PMC article. Review.
-
Mitochondrial-Mediated Oxidative Ca2+/Calmodulin-Dependent Kinase II Activation Induces Early Afterdepolarizations in Guinea Pig Cardiomyocytes: An In Silico Study.J Am Heart Assoc. 2018 Aug 7;7(15):e008939. doi: 10.1161/JAHA.118.008939. J Am Heart Assoc. 2018. PMID: 30371234 Free PMC article.
-
Metabolic rescue ameliorates mitochondrial encephalo-cardiomyopathy in murine and human iPSC models of Leigh syndrome.Clin Transl Med. 2022 Jul;12(7):e954. doi: 10.1002/ctm2.954. Clin Transl Med. 2022. PMID: 35872650 Free PMC article.
References
-
- London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, Viswanathan PC, Pfahnl AE, Shang LL, Madhusudanan M, Baty CJ, Lagana S, Aleong R, Gutmann R, Ackerman MJ, McNamara DM, Weiss R, Dudley SC., Jr Mutation in glycerol-3-phosphate dehydrogenase 1-like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116:2260–8. - PMC - PubMed
-
- Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue: roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res. 1997;81:727–41. - PubMed
-
- Shimizu W, Aiba T, Kamakura S. Mechanisms of disease: current understanding and future challenges in Brugada syndrome. Nat Clin Pract Cardiovasc Med. 2005;2:408–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

