Autophagic degradation of active caspase-8: a crosstalk mechanism between autophagy and apoptosis
- PMID: 20724831
- PMCID: PMC3039736
- DOI: 10.4161/auto.6.7.13038
Autophagic degradation of active caspase-8: a crosstalk mechanism between autophagy and apoptosis
Abstract
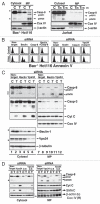
Apoptotic defects endow tumor cells with survival advantages. Such defects allow the cellular stress response to take the path of cytoprotective autophagy, which either precedes or effectively blocks an apoptotic cascade. Inhibition of the cytoprotective autophagic response shifts the cells toward apoptosis, by interfering with an underlying molecular mechanism of cytoprotection. The current study has identified such a mechanism that is centered on the regulation of caspase-8 activity. The study took advantage of Bax(-/-) Hct116 cells that are TRAIL-resistant despite significant DISC processing of caspase-8, and of the availability of a caspase-8-specific antibody that exclusively detects the caspase-8 large subunit or its processed precursor. Utilizing these biological tools, we investigated the expression pattern and subcellular localization of active caspase-8 in TRAIL-mediated autophagy and in the autophagy-to-apoptosis shift upon autophagy inhibition. Our results suggest that the TRAIL-mediated autophagic response counter-balances the TRAIL-mediated apoptotic response by the continuous sequestration of the large caspase-8 subunit in autophagosomes and its subsequent elimination in lysosomes. The current findings are the first to provide evidence for regulation of caspase activity by autophagy and thus broaden the molecular basis for the observed polarization between autophagy and apoptosis.
Figures





References
-
- Salvesen GS, Riedl SJ. Caspase mechanisms. Adv Exp Med Biol. 2008;615:13–23. - PubMed
-
- Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. - PubMed
-
- Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
