Nanoimaging for prion related diseases
- PMID: 20724837
- PMCID: PMC3268959
- DOI: 10.4161/pri.4.4.13125
Nanoimaging for prion related diseases
Abstract
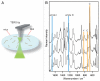
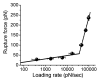
Misfolding and aggregation of prion proteins is linked to a number of neurodegenerative disorders such as Creutzfeldt-Jacob disease (CJD) and its variants: Kuru, Gerstmann-Straussler-Scheinker syndrome and fatal familial insomnia. In prion diseases, infectious particles are proteins that propagate by transmitting a misfolded state of a protein, leading to the formation of aggregates and ultimately to neurodegeneration. Prion phenomenon is not restricted to humans. There are a number of prion-related diseases in a variety of mammals, including bovine spongiform encephalopathy (BSE, also known as "mad cow disease") in cattle. All known prion diseases, collectively called transmissible spongiform encephalopathies (TSEs), are untreatable and fatal. Prion proteins were also found in some fungi where they are responsible for heritable traits. Prion proteins in fungi are easily accessible and provide a powerful model for understanding the general principles of prion phenomenon and molecular mechanisms of mammalian prion diseases. Presently, several fundamental questions related to prions remain unanswered. For example, it is not clear how prions cause the disease. Other unknowns include the nature and structure of infectious agent and how prions replicate. Generally, the phenomenon of misfolding of the prion protein into infectious conformations that have the ability to propagate their properties via aggregation is of significant interest. Despite the crucial importance of misfolding and aggregation, very little is currently known about the molecular mechanisms of these processes. While there is an apparent critical need to study molecular mechanisms underlying misfolding and aggregation, the detailed characterization of these single molecule processes is hindered by the limitation of conventional methods. Although some issues remain unresolved, much progress has been recently made primarily due to the application of nanoimaging tools. The use of nanoimaging methods shows great promise for understanding the molecular mechanisms of prion phenomenon, possibly leading toward early diagnosis and effective treatment of these devastating diseases. This review article summarizes recent reports which advanced our understanding of the prion phenomenon through the use of nanoimaging methods.
Figures

Similar articles
-
Human transmissible spongiform encephalopathies: historic view.Handb Clin Neurol. 2018;153:1-17. doi: 10.1016/B978-0-444-63945-5.00001-5. Handb Clin Neurol. 2018. PMID: 29887130 Review.
-
Prion disease: a deadly disease for protein misfolding.Curr Pharm Biotechnol. 2005 Apr;6(2):167-77. doi: 10.2174/1389201053642321. Curr Pharm Biotechnol. 2005. PMID: 15853695 Review.
-
The transmissible spongiform encephalopathies of livestock.ILAR J. 2015;56(1):7-25. doi: 10.1093/ilar/ilv008. ILAR J. 2015. PMID: 25991695 Review.
-
Molecular biology and pathogenesis of prion diseases.Trends Biochem Sci. 1996 Dec;21(12):482-7. doi: 10.1016/s0968-0004(96)10063-3. Trends Biochem Sci. 1996. PMID: 9009832 Review.
-
[Transmission of spongiform encephalopathies (prion diseases)].HNO. 2002 Apr;50(4):316-26. doi: 10.1007/s00106-002-0652-6. HNO. 2002. PMID: 12063689 Review. German.
Cited by
-
Effect of electrostatics on aggregation of prion protein Sup35 peptide.J Phys Condens Matter. 2012 Apr 25;24(16):164205. doi: 10.1088/0953-8984/24/16/164205. Epub 2012 Mar 30. J Phys Condens Matter. 2012. PMID: 22466073 Free PMC article.
-
Spatially resolved spectroscopic differentiation of hydrophilic and hydrophobic domains on individual insulin amyloid fibrils.Sci Rep. 2016 Sep 21;6:33575. doi: 10.1038/srep33575. Sci Rep. 2016. PMID: 27650589 Free PMC article.
-
Nanoimaging for Molecular Pharmaceutics of Alzheimer's and other Neurodegenerative Disorders.J Mol Pharm Org Process Res. 2013;1:1000e107. doi: 10.4172/jmpopr.1000e107. J Mol Pharm Org Process Res. 2013. PMID: 25584360 Free PMC article. No abstract available.
-
Using NMR spectroscopy to investigate the role played by copper in prion diseases.Neurol Sci. 2020 Sep;41(9):2389-2406. doi: 10.1007/s10072-020-04321-9. Epub 2020 Apr 24. Neurol Sci. 2020. PMID: 32328835 Free PMC article. Review.
-
From Research to Diagnostic Application of Raman Spectroscopy in Neurosciences: Past and Perspectives.Free Neuropathol. 2022 Aug 5;3:19. doi: 10.17879/freeneuropathology-2022-4210. eCollection 2022 Jan. Free Neuropathol. 2022. PMID: 37284145 Free PMC article.
References
-
- Collinge J, Clarke AR. A general model of prion strains and their pathogenicity. Science. 2007;318:930–936. - PubMed
-
- Baxa U, Wickner RB, Steven AC, Anderson DE, Marekov LN, Yau WM, et al. Characterization of beta-sheet structure in Ure2p1–89 yeast prion fibrils by solid-state nuclear magnetic resonance. Biochemistry. 2007;46:13149–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
