Mitochondrial shape changes: orchestrating cell pathophysiology
- PMID: 20725092
- PMCID: PMC2933866
- DOI: 10.1038/embor.2010.115
Mitochondrial shape changes: orchestrating cell pathophysiology
Abstract
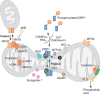
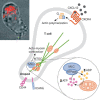
Mitochondria are highly dynamic organelles, the location, size and distribution of which are controlled by a family of proteins that modulate mitochondrial fusion and fission. Recent evidence indicates that mitochondrial morphology is crucial for cell physiology, as changes in mitochondrial shape have been linked to neurodegeneration, calcium signalling, lifespan and cell death. Because immune cells contain few mitochondria, these organelles have been considered to have only a marginal role in this physiological context-which is conversely well characterized from the point of view of signalling. Nevertheless, accumulating evidence shows that mitochondrial dynamics have an impact on the migration and activation of immune cells and on the innate immune response. Here, we discuss the roles of mitochondrial dynamics in cell pathophysiology and consider how studying dynamics in the context of the immune system could increase our knowledge about the role of dynamics in key signalling cascades.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Abarca-Rojano E, Muniz-Hernandez S, Moreno-Altamirano MM, Mondragon-Flores R, Enriquez-Rincon F, Sanchez-Garcia FJ (2009) Re-organization of mitochondria at the NK cell immune synapse. Immunol Lett 122: 18–25 - PubMed
-
- Alexander C et al. (2000) OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26: 211–215 - PubMed
-
- Autret A, Martin SJ (2009) Emerging role for members of the Bcl-2 family in mitochondrial morphogenesis. Mol Cell 36: 355–363 - PubMed
-
- Benard G, Rossignol R (2008) Mitochondrial fluidity matters. Focus on ‘Inherited complex I deficiency is associated with faster protein diffusion in the matrix of moving mitochondria'. Am J Physiol Cell Physiol 294: C1123 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

