Susceptibility of breast cancer cells to an oncolytic matrix (M) protein mutant of vesicular stomatitis virus
- PMID: 20725101
- PMCID: PMC3682428
- DOI: 10.1038/cgt.2010.46
Susceptibility of breast cancer cells to an oncolytic matrix (M) protein mutant of vesicular stomatitis virus
Abstract
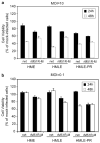
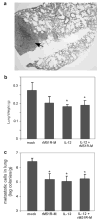
Matrix (M) protein mutants of vesicular stomatitis virus (VSV), such as rM51R-M virus, are attractive candidates as oncolytic viruses for tumor therapies because of their capacity to selectively target cancer cells. The effectiveness of rM51R-M virus as an antitumor agent for the treatment of breast cancer was assessed by determining the ability of rM51R-M virus to infect and kill breast cancer cells in vitro and in vivo. Several human- and mouse-derived breast cancer cell lines were susceptible to infection and killing by rM51R-M virus. Importantly, non-tumorigenic cell lines from normal mammary tissues were also sensitive to VSV infection suggesting that oncogenic transformation does not alter the susceptibility of breast cancer cells to oncolytic VSV. In contrast to results obtained in vitro, rM51R-M virus was only partially effective at inducing regression of primary breast tumors in vivo. Furthermore, we were unable to induce complete regression of the primary and metastatic tumors when tumor-bearing mice were treated with a vector expressing interleukin (IL)-12 or a combination of rM51R-M virus and IL-12. Our results indicate that although breast cancer cells may be susceptible to VSV in vitro, more aggressive treatment combinations are required to effectively treat both local and metastatic breast cancers in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses.Virology. 2004 Dec 5;330(1):34-49. doi: 10.1016/j.virol.2004.08.039. Virology. 2004. PMID: 15527832
-
Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis.J Immunother Cancer. 2021 Mar;9(3):e002096. doi: 10.1136/jitc-2020-002096. J Immunother Cancer. 2021. PMID: 33722907 Free PMC article.
-
Vesicular Stomatitis Virus (VSV) G Glycoprotein Can Be Modified to Create a Her2/Neu-Targeted VSV That Eliminates Large Implanted Mammary Tumors.J Virol. 2023 Jun 29;97(6):e0037223. doi: 10.1128/jvi.00372-23. Epub 2023 May 18. J Virol. 2023. PMID: 37199666 Free PMC article.
-
[Vesicular stomatitis virus in the fight against cancer].Med Sci (Paris). 2013 Feb;29(2):175-82. doi: 10.1051/medsci/2013292015. Epub 2013 Feb 28. Med Sci (Paris). 2013. PMID: 23452604 Review. French.
-
Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer.J Gen Virol. 2012 Dec;93(Pt 12):2529-2545. doi: 10.1099/vir.0.046672-0. Epub 2012 Oct 10. J Gen Virol. 2012. PMID: 23052398 Free PMC article. Review.
Cited by
-
Murine Tumor Models for Oncolytic Rhabdo-Virotherapy.ILAR J. 2016;57(1):73-85. doi: 10.1093/ilar/ilv048. ILAR J. 2016. PMID: 27034397 Free PMC article.
-
Development of COVID-19 Vaccine Candidates Using Attenuated Recombinant Vesicular Stomatitis Virus Vectors with M Protein Mutations.Viruses. 2025 Jul 30;17(8):1062. doi: 10.3390/v17081062. Viruses. 2025. PMID: 40872776 Free PMC article.
-
Vesicular stomatitis virus as an oncolytic agent against pancreatic ductal adenocarcinoma.J Virol. 2012 Mar;86(6):3073-87. doi: 10.1128/JVI.05640-11. Epub 2012 Jan 11. J Virol. 2012. PMID: 22238308 Free PMC article.
-
Oncolytic Virotherapy for Breast Cancer Treatment.Curr Gene Ther. 2018;18(4):192-205. doi: 10.2174/1566523218666180910163805. Curr Gene Ther. 2018. PMID: 30207220 Free PMC article. Review.
-
Evaluation of vesicular stomatitis virus mutant as an oncolytic agent against prostate cancer.Int J Clin Exp Med. 2014 May 15;7(5):1204-13. eCollection 2014. Int J Clin Exp Med. 2014. PMID: 24995075 Free PMC article.
References
-
- Dodwell D, Williamson D. Beyond tamoxifen: extended and late extended endocrine therapy in postmenopausal early breast cancer. Cancer Treat Rev. 2008;34:137–144. - PubMed
-
- Nicolini A, Giardino R, Carpi A, Ferrari P, Anselmi L, Colosimo S. Metastatic breast cancer: an updating. Biomed Pharmacother. 2006;60:548–556. - PubMed
-
- Ahmed M, Cramer SD, Lyles DS. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology. 2004;330:34–49. - PubMed
-
- Balachandran S, Barber GN. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life. 2000;50:135–138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

