PAF-AH Catalytic Subunits Modulate the Wnt Pathway in Developing GABAergic Neurons
- PMID: 20725507
- PMCID: PMC2901149
- DOI: 10.3389/fncel.2010.00019
PAF-AH Catalytic Subunits Modulate the Wnt Pathway in Developing GABAergic Neurons
Abstract
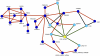
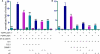
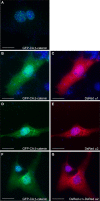
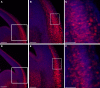
Platelet-activating factor acetylhydrolase 1B (PAF-AH) inactivates the potent phospholipid platelet-activating factor (PAF) and is composed of two catalytic subunits (alpha1 and alpha2) and a dimeric regulatory subunit, LIS1. The function of the catalytic subunits in brain development remains unknown. Here we examined their effects on proliferation in the ganglionic eminences and tangential migration. In alpha1 and alpha2 catalytic subunits knockout mice we noticed an increase in the size of the ganglionic eminences resulting from increased proliferation of GABAergic neurons. Our results indicate that the catalytic subunits act as negative regulators of the Wnt signaling pathway. Overexpression of each of the PAF-AH catalytic subunits reduced the amount of nuclear beta-catenin and provoked a shift of this protein from the nucleus to the cytoplasm. In the double mutant mice, Wnt signaling increased in the ganglionic eminences and in the dorsal part of the cerebral cortex. In situ hybridization revealed increased and expanded expression of a downstream target of the Wnt pathway (Cyclin D1), and of upstream Wnt components (Tcf4, Tcf3 and Wnt7B). Furthermore, the interneurons in the cerebral cortex were more numerous and in a more advanced position. Transplantation assays revealed a non-cell autonomous component to this phenotype, which may be explained in part by increased and expanded expression of Sdf1 and Netrin-1. Our findings strongly suggest that PAF-AH catalytic subunits modulate the Wnt pathway in restricted areas of the developing cerebral cortex. We hypothesize that modulation of the Wnt pathway is the evolutionary conserved activity of the PAF-AH catalytic subunits.
Keywords: Wnt; beta-catenin; ganglionic eminences; platelet-activating factor acetylhydrolase IB.
Figures

Similar articles
-
A Complex of Type I Platelet-Activating Factor Acetylhydrolase (PAF-AH) Catalytic Subunits Switches from α1/α2 Heterodimer to α2/α2 Homodimer during Adipocyte Differentiation of 3T3-L1 Cells.Biol Pharm Bull. 2023;46(2):257-262. doi: 10.1248/bpb.b22-00666. Biol Pharm Bull. 2023. PMID: 36724953
-
Platelet-Activating Factor Acetylhydrolase Expression in BRCA1 Mutant Ovarian Cancer as a Protective Factor and Potential Negative Regulator of the Wnt Signaling Pathway.Biomedicines. 2021 Jun 22;9(7):706. doi: 10.3390/biomedicines9070706. Biomedicines. 2021. PMID: 34206491 Free PMC article.
-
Intracellular PAF-Acetylhydrolase Type I.Enzymes. 2015;38:23-36. doi: 10.1016/bs.enz.2015.09.007. Epub 2015 Nov 4. Enzymes. 2015. PMID: 26612644
-
Platelet-activating factor acetylhydrolase.Prostaglandins Other Lipid Mediat. 2002 Aug;68-69:83-94. doi: 10.1016/s0090-6980(02)00023-0. Prostaglandins Other Lipid Mediat. 2002. PMID: 12432911 Review.
-
Platelet-activating factor acetylhydrolase (PAF-AH).J Biochem. 2002 May;131(5):635-40. doi: 10.1093/oxfordjournals.jbchem.a003145. J Biochem. 2002. PMID: 11983068 Review.
Cited by
-
Comprehensive Analysis of the Prognostic and Immunological Role of PAFAH1B in Pan-Cancer.Front Mol Biosci. 2022 Feb 3;8:799497. doi: 10.3389/fmolb.2021.799497. eCollection 2021. Front Mol Biosci. 2022. PMID: 35187070 Free PMC article.
-
Aberrant expression of PAFAH1B3 associates with poor prognosis and affects proliferation and aggressiveness in hypopharyngeal squamous cell carcinoma.Onco Targets Ther. 2019 Apr 11;12:2799-2808. doi: 10.2147/OTT.S196324. eCollection 2019. Onco Targets Ther. 2019. PMID: 31043794 Free PMC article.
-
Lipoquality control by phospholipase A2 enzymes.Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(9):677-702. doi: 10.2183/pjab.93.043. Proc Jpn Acad Ser B Phys Biol Sci. 2017. PMID: 29129849 Free PMC article. Review.
-
Loss of PAFAH1B2 reduces amyloid-β generation by promoting the degradation of amyloid precursor protein C-terminal fragments.J Neurosci. 2012 Dec 12;32(50):18204-14. doi: 10.1523/JNEUROSCI.2681-12.2012. J Neurosci. 2012. PMID: 23238734 Free PMC article.
-
Dkk2/Frzb in the dermal papillae regulates feather regeneration.Dev Biol. 2014 Mar 15;387(2):167-78. doi: 10.1016/j.ydbio.2014.01.010. Epub 2014 Jan 21. Dev Biol. 2014. PMID: 24463139 Free PMC article.
References
-
- Alcantara S., Ruiz M., De Castro F., Soriano E., Sotelo C. (2000). Netrin 1 acts as an attractive or as a repulsive cue for distinct migrating neurons during the development of the cerebellar system. Development 127, 1359–1372 - PubMed
-
- Andrews W., Barber M., Hernadez-Miranda L. R., Xian J., Rakic S., Sundaresan V., Rabbitts T. H., Pannell R., Rabbitts P., Thompson H., Erskine L., Murakami F., Parnavelas J. G. (2008). The role of Slit-Robo signaling in the generation, migration and morphological differentiation of cortical interneurons. Dev. Biol. 313, 648–65810.1016/j.ydbio.2007.10.052 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

