Do different neurons age differently? Direct genome-wide analysis of aging in single identified cholinergic neurons
- PMID: 20725513
- PMCID: PMC2910937
- DOI: 10.3389/neuro.24.006.2010
Do different neurons age differently? Direct genome-wide analysis of aging in single identified cholinergic neurons
Abstract
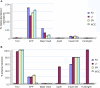
Aplysia californica is a powerful experimental system to study the entire scope of genomic and epigenomic regulation at the resolution of single functionally characterized neurons and is an emerging model in the neurobiology of aging. First, we have identified and cloned a number of evolutionarily conserved genes that are age-related, including components of apoptosis and chromatin remodeling. Second, we performed gene expression profiling of different identified cholinergic neurons between young and aged animals. Our initial analysis indicates that two cholinergic neurons (R2 and LPl1) revealed highly differential genome-wide changes following aging suggesting that on the molecular scale different neurons indeed age differently. Each of the neurons tested has a unique subset of genes differentially expressed in older animals, and the majority of differently expressed genes (including those related to apoptosis and Alzheimer's disease) are found in aging neurons of one but not another type. The performed analysis allows us to implicate (i) cell specific changes in histones, (ii) DNA methylation and (iii) regional relocation of RNAs as key processes underlying age-related changes in neuronal functions and synaptic plasticity. These mechanisms can fine-tune the dynamics of long-term chromatin remodeling, or control weakening and the loss of synaptic connections in aging. At the same time our genomic tests revealed evolutionarily conserved gene clusters associated with aging (e.g., apoptosis-, telomere- and redox-dependent processes, insulin and estrogen signaling and water channels).
Keywords: Alzheimer's disease; Aplysia; epigenetics of aging; histones; single neuron transcriptome.
Figures








References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

