Peptide-Like Molecules (PLMs): A Journey from Peptide Bond Isosteres to Gramicidin S Mimetics and Mitochondrial Targeting Agents
- PMID: 20725595
- PMCID: PMC2922051
- DOI: 10.2533/chimia.2009.764
Peptide-Like Molecules (PLMs): A Journey from Peptide Bond Isosteres to Gramicidin S Mimetics and Mitochondrial Targeting Agents
Abstract
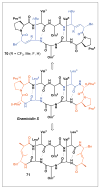
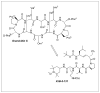
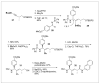
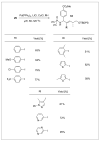
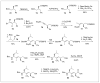
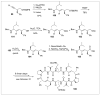
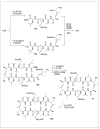
Peptides are natural ligands and substrates for receptors and enzymes and exhibit broad physiological effects. However, their use as therapeutic agents often suffers from poor bioavailability and insufficient membrane permeability. The success of peptide mimicry hinges on the ability of bioisosteres, in particular peptide bond replacements, to adopt suitable secondary structures relevant to peptide strands and position functional groups in equivalent space. This perspective highlights past and ongoing studies in our group that involve new methods development as well as specific synthetic library preparations and applications in chemical biology, with the goal to enhance the use of alkene and cyclopropane peptide bond isosteres.
Figures




















Similar articles
-
Electrostatic versus steric effects in peptidomimicry: synthesis and secondary structure analysis of gramicidin S analogues with (E)-alkene peptide isosteres.J Am Chem Soc. 2005 Apr 27;127(16):5742-3. doi: 10.1021/ja051002s. J Am Chem Soc. 2005. PMID: 15839644
-
Allylic Amines as Key Building Blocks in the Synthesis of (E)-Alkene Peptide Isosteres.Org Process Res Dev. 2012;16(1):26-34. doi: 10.1021/op2002613. Org Process Res Dev. 2012. PMID: 22323894 Free PMC article.
-
Convergent approach to (E)-alkene and cyclopropane peptide isosteres.Org Lett. 2005 Jan 6;7(1):103-6. doi: 10.1021/ol0477529. Org Lett. 2005. PMID: 15624988
-
An evaluation of peptide-bond isosteres.Chembiochem. 2011 Aug 16;12(12):1801-7. doi: 10.1002/cbic.201100272. Epub 2011 Jul 12. Chembiochem. 2011. PMID: 21751326 Free PMC article. Review.
-
Towards protein surface mimetics.Curr Med Chem. 1998 Feb;5(1):29-62. Curr Med Chem. 1998. PMID: 9481033 Review.
Cited by
-
Synthesis and Evaluation of a Mitochondria-Targeting Poly(ADP-ribose) Polymerase-1 Inhibitor.ACS Chem Biol. 2018 Oct 19;13(10):2868-2879. doi: 10.1021/acschembio.8b00423. Epub 2018 Sep 14. ACS Chem Biol. 2018. PMID: 30184433 Free PMC article.
-
Structural consequences of beta-amino acid preorganization in a self-assembling alpha/beta-peptide: fundamental studies of foldameric helix bundles.J Am Chem Soc. 2010 Sep 8;132(35):12378-87. doi: 10.1021/ja103543s. J Am Chem Soc. 2010. PMID: 20718422 Free PMC article.
-
Stereoselective synthesis of Gly-Gly-type (E)-methylalkene and (Z)-chloroalkene dipeptide isosteres and their application to 14-mer RGG peptidomimetics.RSC Adv. 2020 Aug 10;10(49):29373-29377. doi: 10.1039/d0ra06554d. eCollection 2020 Aug 5. RSC Adv. 2020. PMID: 35521116 Free PMC article.
-
Oligo-Quinolylene-Vinylene Foldamers.Chemistry. 2021 Jan 13;27(3):1031-1038. doi: 10.1002/chem.202003559. Epub 2020 Dec 3. Chemistry. 2021. PMID: 32881144 Free PMC article.
-
Small Molecule Inhibitors of the PCSK9·LDLR Interaction.J Am Chem Soc. 2018 Mar 7;140(9):3242-3249. doi: 10.1021/jacs.7b09360. Epub 2018 Feb 26. J Am Chem Soc. 2018. PMID: 29378408 Free PMC article.
References
-
- Yang W, Lu W, Lu Y, Zhong M, Sun J, Thomas AE, Wilkinson JM, Fucini RV, Lam M, Randal M, Shi XP, Jacobs JW, McDowell RS, Gordon EM, Ballinger MD. J Med Chem. 2006;49:839. - PubMed
-
- Li CS, Deschenes D, Desmarais S, Falgueyret JP, Gauthier JY, Kimmel DB, Leger S, Masse F, McGrath ME, McKay DJ, Percival MD, Riendeau D, Rodan SB, Therien M, Truong VL, Wesolowski G, Zamboni R, Black WC. Bioorg Med Chem Lett. 2006;16:1985. - PubMed
-
- Zanda M. New J Chem. 2004;28:1401.
-
- Oishi S, Miyamoto K, Niida A, Yamamoto M, Ajito K, Tamamura H, Otaka A, Kuroda Y, Asai A, Fujii N. Tetrahedron. 2006;62:1416.
-
- Kim J, Hewitt G, Carroll P, Sieburth SM. J Org Chem. 2005;70:5781. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

