DNA mismatch repair in eukaryotes and bacteria
- PMID: 20725617
- PMCID: PMC2915661
- DOI: 10.4061/2010/260512
DNA mismatch repair in eukaryotes and bacteria
Abstract
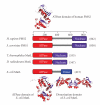
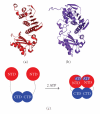
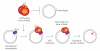
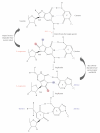
DNA mismatch repair (MMR) corrects mismatched base pairs mainly caused by DNA replication errors. The fundamental mechanisms and proteins involved in the early reactions of MMR are highly conserved in almost all organisms ranging from bacteria to human. The significance of this repair system is also indicated by the fact that defects in MMR cause human hereditary nonpolyposis colon cancers as well as sporadic tumors. To date, 2 types of MMRs are known: the human type and Escherichia coli type. The basic features of the former system are expected to be universal among the vast majority of organisms including most bacteria. Here, I review the molecular mechanisms of eukaryotic and bacterial MMR, emphasizing on the similarities between them.
Figures


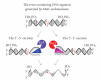

References
-
- Friedberg EC, Walker GC, Siede W. DNA Repair and Mutagenesis. 2nd edition. Washington, DC, USA: American Society for Microbiology; 2005.
-
- Schaaper RM. Base selection, proofreading, and mismatch repair during DNA replication in Escherichia coli . Journal of Biological Chemistry. 1993;268(32):23762–23765. - PubMed
-
- Fishel R, Kolodner RD. Identification of mismatch repair genes and their role in the development of cancer. Current Opinion in Genetics and Development. 1995;5(3):382–395. - PubMed
-
- Suter CM, Martin DIK, Ward RL. Germline epimutation of MLH1 in individuals with multiple cancers. Nature Genetics. 2004;36(5):497–501. - PubMed
-
- Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Research. 1997;57(5):808–811. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

