"One Ring to Bind Them All"-Part II: Identification of Promising G-Quadruplex Ligands by Screening of Cyclophane-Type Macrocycles
- PMID: 20725622
- PMCID: PMC2915812
- DOI: 10.4061/2010/460561
"One Ring to Bind Them All"-Part II: Identification of Promising G-Quadruplex Ligands by Screening of Cyclophane-Type Macrocycles
Abstract
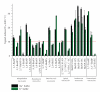
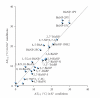
A collection of 26 polyammonium cyclophane-type macrocycles with a large structural diversity has been screened for G-quadruplex recognition. A two-step selection procedure based on the FRET-melting assay was carried out enabling identification of macrocycles of high affinity (DeltaT(1/2) up to 30 degrees C) and high selectivity for the human telomeric G-quadruplex. The four selected hits possess sophisticated architectures, more particularly the presence of a pendant side-arm as well as the existence of a particular topological arrangement appear to be strong determinants of quadruplex binding. These compounds are thus likely to create multiple contacts with the target that may be at the origin of their high selectivity, thereby suggesting that this class of macrocycles offers unique advantages for targeting G-quadruplex-DNA.
Figures





Similar articles
-
Recognition of G-quadruplex DNA by triangular star-shaped compounds: with or without side chains?Chemistry. 2011 Apr 11;17(16):4529-39. doi: 10.1002/chem.201002810. Epub 2011 Mar 17. Chemistry. 2011. PMID: 21416510
-
"One ring to bind them all"-part I: the efficiency of the macrocyclic scaffold for g-quadruplex DNA recognition.J Nucleic Acids. 2010 May 24;2010:525862. doi: 10.4061/2010/525862. J Nucleic Acids. 2010. PMID: 20725629 Free PMC article.
-
Structural basis for telomeric G-quadruplex targeting by naphthalene diimide ligands.J Am Chem Soc. 2012 Feb 8;134(5):2723-31. doi: 10.1021/ja2102423. Epub 2012 Jan 31. J Am Chem Soc. 2012. PMID: 22280460
-
Methods for investigating G-quadruplex DNA/ligand interactions.Chem Soc Rev. 2011 Nov;40(11):5293-307. doi: 10.1039/c1cs15117g. Epub 2011 Jul 1. Chem Soc Rev. 2011. PMID: 21720638 Review.
-
Physiological relevance of telomeric G-quadruplex formation: a potential drug target.Bioessays. 2007 Feb;29(2):155-65. doi: 10.1002/bies.20523. Bioessays. 2007. PMID: 17226803 Review.
Cited by
-
Bis-indole derivatives with antitumor activity turn out to be specific ligands of human telomeric G-quadruplex.Front Chem. 2014 Jul 24;2:54. doi: 10.3389/fchem.2014.00054. eCollection 2014. Front Chem. 2014. PMID: 25105115 Free PMC article.
-
Dual targeting of higher-order DNA structures by azacryptands induces DNA junction-mediated DNA damage in cancer cells.Nucleic Acids Res. 2021 Oct 11;49(18):10275-10288. doi: 10.1093/nar/gkab796. Nucleic Acids Res. 2021. PMID: 34551430 Free PMC article.
-
TWJ-Screen: an isothermal screening assay to assess ligand/DNA junction interactions in vitro.Nucleic Acids Res. 2018 Feb 16;46(3):e16. doi: 10.1093/nar/gkx1118. Nucleic Acids Res. 2018. PMID: 29149299 Free PMC article.
-
Deciphering the Interplay Between G-Quadruplexes and Natural/Synthetic Polyamines.Chembiochem. 2025 Apr 1;26(7):e202400873. doi: 10.1002/cbic.202400873. Epub 2024 Dec 23. Chembiochem. 2025. PMID: 39656761 Free PMC article. Review.
-
HIV-1 nucleocapsid protein increases strand transfer recombination by promoting dimeric G-quartet formation.J Biol Chem. 2011 Aug 26;286(34):29838-47. doi: 10.1074/jbc.M111.262352. Epub 2011 Jul 7. J Biol Chem. 2011. PMID: 21737842 Free PMC article.
References
-
- Monchaud D, Teulade-Fichou M-P. A hitchhiker’s guide to G-quadruplex ligands. Organic and Biomolecular Chemistry. 2008;6(4):627–636. - PubMed
-
- Arola A, Vilar R. Stabilisation of G-quadruplex DNA by small molecules. Current Topics in Medicinal Chemistry. 2008;8(15):1405–1415. - PubMed
-
- Franceschin M. G-quadruplex DNA structures and organic chemistry: more than one connection. European Journal of Organic Chemistry. 2009;(14):2225–2238.
-
- Neidle S. The structures of quadruplex nucleic acids and their drug complexes. Current Opinion in Structural Biology. 2009;19(3):239–250. - PubMed
-
- Shin-ya K, Wierzba K, Matsuo K, et al. Telomestatin, a novel telomerase inhibitor from Streptomyces anulatus. Journal of the American Chemical Society. 2001;123(6):1262–1263. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

