Synthesis and g-quadruplex-binding properties of defined acridine oligomers
- PMID: 20725626
- PMCID: PMC2915818
- DOI: 10.4061/2010/489060
Synthesis and g-quadruplex-binding properties of defined acridine oligomers
Abstract
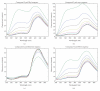
The synthesis of oligomers containing two or three acridine units linked through 2-aminoethylglycine using solid-phase methodology is described. Subsequent studies on cell viability showed that these compounds are not cytotoxic. Binding to several DNA structures was studied by competitive dialysis, which showed a clear affinity for DNA sequences that form G-quadruplexes and parallel triplexes. The fluorescence spectra of acridine oligomers were affected strongly upon binding to DNA. These spectral changes were used to calculate the binding constants (K). Log K were found to be in the order of 4-6.
Figures

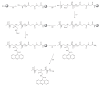

Similar articles
-
A comparative study on the interaction of acridine and synthetic bis-acridine with G-quadruplex structure.J Biochem Biophys Methods. 2003 Jul 31;57(1):65-74. doi: 10.1016/s0165-022x(03)00081-2. J Biochem Biophys Methods. 2003. PMID: 12834964
-
Acridine and quindoline oligomers linked through a 4-aminoproline backbone prefer G-quadruplex structures.Biochim Biophys Acta. 2011 Aug;1810(8):769-76. doi: 10.1016/j.bbagen.2011.04.013. Epub 2011 May 5. Biochim Biophys Acta. 2011. PMID: 21570448
-
Isolation and characterization of a monoclonal anti-quadruplex DNA antibody from autoimmune "viable motheaten" mice.Biochemistry. 1998 Nov 17;37(46):16325-37. doi: 10.1021/bi981354u. Biochemistry. 1998. PMID: 9819225
-
Stability and structure of model DNA triplexes and quadruplexes and their interactions with small ligands.Prog Nucleic Acid Res Mol Biol. 1998;59:55-94. doi: 10.1016/s0079-6603(08)61029-6. Prog Nucleic Acid Res Mol Biol. 1998. PMID: 9427840 Review.
-
Higher-order quadruplex structures.Top Curr Chem. 2013;330:23-46. doi: 10.1007/128_2012_350. Top Curr Chem. 2013. PMID: 22790417 Review.
Cited by
-
Practical Microwave Synthesis of Carbazole Aldehydes for the Development of DNA-Binding Ligands.Molecules. 2019 Mar 9;24(5):965. doi: 10.3390/molecules24050965. Molecules. 2019. PMID: 30857275 Free PMC article.
-
Synthesis, DNA-binding and antiproliferative properties of acridine and 5-methylacridine derivatives.Molecules. 2012 Jun 8;17(6):7067-82. doi: 10.3390/molecules17067067. Molecules. 2012. PMID: 22683895 Free PMC article.
-
Structure and stability of human telomeric G-quadruplex with preclinical 9-amino acridines.PLoS One. 2013;8(3):e57701. doi: 10.1371/journal.pone.0057701. Epub 2013 Mar 15. PLoS One. 2013. PMID: 23554865 Free PMC article.
References
-
- Palchaudhuri R, Hergenrother PJ. DNA as a target for anticancer compounds: methods to determine the mode of binding and the mechanism of action. Current Opinion in Biotechnology. 2007;18(6):497–503. - PubMed
-
- Parkinson GN, Lee MPH, Neidle S. Crystal structure of parallel quadruplexes from human telomeric DNA. Nature. 2002;417(6891):876–880. - PubMed
-
- Huppert JL. Hunting G-quadruplexes. Biochimie. 2008;90(8):1140–1148. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

