The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation
- PMID: 20725905
- PMCID: PMC2967616
- DOI: 10.1002/hep.23848
The oncogenic effect of sulfatase 2 in human hepatocellular carcinoma is mediated in part by glypican 3-dependent Wnt activation
Abstract
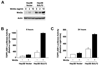
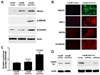
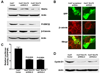
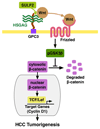
Heparan sulfate proteoglycans (HSPGs) act as coreceptors or storage sites for growth factors and cytokines such as fibroblast growth factor and Wnts. Glypican 3 (GPC3) is the most highly expressed HSPG in hepatocellular carcinoma (HCC). Sulfatase 2 (SULF2), an enzyme with 6-O-desulfatase activity on HSPGs, is up-regulated in 60% of primary HCCs and is associated with a worse prognosis. We have previously shown that the oncogenic effect of SULF2 in HCC may be mediated in part through up-regulation of GPC3. Here we demonstrate that GPC3 stimulates the Wnt/β-catenin pathway and mediates the oncogenic function of SULF2 in HCC. Wnt signaling in vitro and in vivo was assessed in SULF2-negative Hep3B HCC cells transfected with SULF2 and in SULF2-expressing Huh7 cells transfected with short hairpin RNA targeting SULF2. The interaction between GPC3, SULF2, and Wnt3a was assessed by coimmunoprecipitation and flow cytometry. β-catenin-dependent transcriptional activity was assessed with the TOPFLASH (T cell factor reporter plasmid) luciferase assay. In HCC cells, SULF2 increased cell surface GPC3 and Wnt3a expression, stabilized β-catenin, and activated T cell factor transcription factor activity and expression of the Wnt/β-catenin target gene cyclin D1. Opposite effects were observed in SULF2-knockdown models. In vivo, nude mouse xenografts established from SULF2-transfected Hep3B cells showed enhanced GPC3, Wnt3a, and β-catenin levels.
Conclusion: Together, these findings identify a novel mechanism mediating the oncogenic function of SULF2 in HCC that includes GPC3-mediated activation of Wnt signaling via the Wnt3a/glycogen synthase kinase 3 beta axis.
Figures




Similar articles
-
Sulfatase 2 up-regulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma.Hepatology. 2008 Apr;47(4):1211-22. doi: 10.1002/hep.22202. Hepatology. 2008. PMID: 18318435 Free PMC article.
-
Sulfatase 2 protects hepatocellular carcinoma cells against apoptosis induced by the PI3K inhibitor LY294002 and ERK and JNK kinase inhibitors.Liver Int. 2010 Nov;30(10):1522-8. doi: 10.1111/j.1478-3231.2010.02336.x. Epub 2010 Sep 8. Liver Int. 2010. PMID: 21040406 Free PMC article.
-
A Frizzled-Like Cysteine-Rich Domain in Glypican-3 Mediates Wnt Binding and Regulates Hepatocellular Carcinoma Tumor Growth in Mice.Hepatology. 2019 Oct;70(4):1231-1245. doi: 10.1002/hep.30646. Epub 2019 May 24. Hepatology. 2019. PMID: 30963603 Free PMC article.
-
Heparin-degrading sulfatases in hepatocellular carcinoma: roles in pathogenesis and therapy targets.Future Oncol. 2008 Dec;4(6):803-14. doi: 10.2217/14796694.4.6.803. Future Oncol. 2008. PMID: 19086847 Free PMC article. Review.
-
Glypican-3 is a prognostic factor and an immunotherapeutic target in hepatocellular carcinoma.World J Gastroenterol. 2016 Jan 7;22(1):275-83. doi: 10.3748/wjg.v22.i1.275. World J Gastroenterol. 2016. PMID: 26755876 Free PMC article. Review.
Cited by
-
Oncogenic Wnt3a expression as an estimable prognostic marker for hepatocellular carcinoma.World J Gastroenterol. 2016 Apr 14;22(14):3829-36. doi: 10.3748/wjg.v22.i14.3829. World J Gastroenterol. 2016. PMID: 27076768 Free PMC article.
-
Sulfatase 2 Affects Polarization of M2 Macrophages through the IL-8/JAK2/STAT3 Pathway in Bladder Cancer.Cancers (Basel). 2022 Dec 26;15(1):131. doi: 10.3390/cancers15010131. Cancers (Basel). 2022. PMID: 36612128 Free PMC article.
-
The Role of Heparanase and Sulfatases in the Modification of Heparan Sulfate Proteoglycans within the Tumor Microenvironment and Opportunities for Novel Cancer Therapeutics.Front Oncol. 2014 Jul 24;4:195. doi: 10.3389/fonc.2014.00195. eCollection 2014. Front Oncol. 2014. PMID: 25105093 Free PMC article. Review.
-
Activation of the transforming growth factor-β/SMAD transcriptional pathway underlies a novel tumor-promoting role of sulfatase 1 in hepatocellular carcinoma.Hepatology. 2015 Apr;61(4):1269-83. doi: 10.1002/hep.27658. Epub 2015 Feb 13. Hepatology. 2015. PMID: 25503294 Free PMC article.
-
Wnt3a: functions and implications in cancer.Chin J Cancer. 2015 Sep 14;34(12):554-62. doi: 10.1186/s40880-015-0052-4. Chin J Cancer. 2015. PMID: 26369691 Free PMC article. Review.
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Roberts LR, Gores GJ. Hepatocellular carcinoma: molecular pathways and new therapeutic targets. Semin Liver Dis. 2005;25:212–225. - PubMed
-
- Lee HC, Kim M, Wands JR. Wnt/Frizzled signaling in hepatocellular carcinoma. Front Biosci. 2006;11:1901–1915. - PubMed
-
- Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res. 2005;65:6245–6254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
