In vitro bypass of the major malondialdehyde- and base propenal-derived DNA adduct by human Y-family DNA polymerases κ, ι, and Rev1
- PMID: 20726503
- PMCID: PMC2943251
- DOI: 10.1021/bi1009024
In vitro bypass of the major malondialdehyde- and base propenal-derived DNA adduct by human Y-family DNA polymerases κ, ι, and Rev1
Abstract
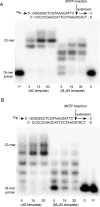
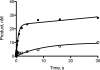
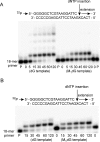
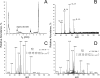
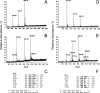
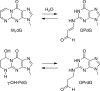
3-(2'-Deoxy-β-d-erythro-pentofuranosyl)pyrimido-[1,2-a]purin-10(3H)-one (M(1)dG) is the major adduct derived from the reaction of DNA with the lipid peroxidation product malondialdehyde and the DNA peroxidation product base propenal. M(1)dG is mutagenic in Escherichia coli and mammalian cells, inducing base-pair substitutions (M(1)dG → A and M(1)dG → T) and frameshift mutations. Y-family polymerases may contribute to the mutations induced by M(1)dG in vivo. Previous reports described the bypass of M(1)dG by DNA polymerases η and Dpo4. The present experiments were conducted to evaluate bypass of M(1)dG by the human Y-family DNA polymerases κ, ι, and Rev1. M(1)dG was incorporated into template-primers containing either dC or dT residues 5' to the adduct, and the template-primers were subjected to in vitro replication by the individual DNA polymerases. Steady-state kinetic analysis of single nucleotide incorporation indicates that dCMP is most frequently inserted by hPol κ opposite the adduct in both sequence contexts, followed by dTMP and dGMP. dCMP and dTMP were most frequently inserted by hPol ι, and only dCMP was inserted by Rev1. hPol κ extended template-primers in the order M(1)dG:dC > M(1)dG:dG > M(1)dG:dT ∼ M(1)dG:dA, but neither hPol ι nor Rev1 extended M(1)dG-containing template-primers. Liquid chromatography-mass spectrometry analysis of the products of hPol κ-catalyzed extension verified this preference in the 3'-GXC-5' template sequence but revealed the generation of a series of complex products in which dAMP is incorporated opposite M(1)dG in the 3'-GXT-5' template sequence. The results indicate that DNA hPol κ or the combined action of hPol ι or Rev1 and hPol κ bypass M(1)dG residues in DNA and generate products that are consistent with some of the mutations induced by M(1)dG in mammalian cells.
Figures

Similar articles
-
Translesion DNA synthesis by human DNA polymerase eta on templates containing a pyrimidopurinone deoxyguanosine adduct, 3-(2'-deoxy-beta-d-erythro-pentofuranosyl)pyrimido-[1,2-a]purin-10(3H)-one.Biochemistry. 2009 Jan 20;48(2):471-80. doi: 10.1021/bi801591a. Biochemistry. 2009. PMID: 19108641 Free PMC article.
-
The peroxidation-derived DNA adduct, 6-oxo-M1dG, is a strong block to replication by human DNA polymerase η.J Biol Chem. 2023 Aug;299(8):105067. doi: 10.1016/j.jbc.2023.105067. Epub 2023 Jul 18. J Biol Chem. 2023. PMID: 37468099 Free PMC article.
-
Structural and functional analysis of Sulfolobus solfataricus Y-family DNA polymerase Dpo4-catalyzed bypass of the malondialdehyde-deoxyguanosine adduct.Biochemistry. 2009 Aug 4;48(30):7079-88. doi: 10.1021/bi9003588. Biochemistry. 2009. PMID: 19492857 Free PMC article.
-
Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal.Chem Res Toxicol. 2009 May;22(5):759-78. doi: 10.1021/tx9000489. Chem Res Toxicol. 2009. PMID: 19397281 Free PMC article. Review.
-
Variations on a theme: eukaryotic Y-family DNA polymerases.Biochim Biophys Acta. 2010 May;1804(5):1113-23. doi: 10.1016/j.bbapap.2009.07.004. Epub 2009 Jul 17. Biochim Biophys Acta. 2010. PMID: 19616647 Free PMC article. Review.
Cited by
-
Synthesis of site-specific DNA-protein conjugates and their effects on DNA replication.ACS Chem Biol. 2014 Aug 15;9(8):1860-8. doi: 10.1021/cb5001795. Epub 2014 Jun 26. ACS Chem Biol. 2014. PMID: 24918113 Free PMC article.
-
Polymerase Bypass of N(6)-Deoxyadenosine Adducts Derived from Epoxide Metabolites of 1,3-Butadiene.Chem Res Toxicol. 2015 Jul 20;28(7):1496-507. doi: 10.1021/acs.chemrestox.5b00166. Epub 2015 Jul 6. Chem Res Toxicol. 2015. PMID: 26098310 Free PMC article.
-
Malondialdehyde Epitopes as Targets of Immunity and the Implications for Atherosclerosis.Adv Immunol. 2016;131:1-59. doi: 10.1016/bs.ai.2016.02.001. Epub 2016 Apr 5. Adv Immunol. 2016. PMID: 27235680 Free PMC article. Review.
-
Site-Specific Synthesis of Oligonucleotides Containing 6-Oxo-M1dG, the Genomic Metabolite of M1dG, and Liquid Chromatography-Tandem Mass Spectrometry Analysis of Its In Vitro Bypass by Human Polymerase ι.Chem Res Toxicol. 2021 Dec 20;34(12):2567-2578. doi: 10.1021/acs.chemrestox.1c00334. Epub 2021 Dec 3. Chem Res Toxicol. 2021. PMID: 34860508 Free PMC article.
-
Protein modification by adenine propenal.Chem Res Toxicol. 2014 Oct 20;27(10):1732-42. doi: 10.1021/tx500218g. Epub 2014 Sep 24. Chem Res Toxicol. 2014. PMID: 25211669 Free PMC article.
References
-
- Marnett L. J.; Plastaras J. P. (2001) Endogenous DNA damage and mutation. Trends Genet. 17, 214–221. - PubMed
-
- Migliore L.; Coppede F. (2002) Genetic and environmental factors in cancer and neurodegenerative diseases. Mutat. Res. 512, 135–153. - PubMed
-
- Olinski R.; Gackowski D.; Foksinski M.; Rozalski R.; Roszkowski K.; Jaruga P. (2002) Oxidative DNA damage: assessment of the role in carcinogenesis, atherosclerosis, and acquired immunodeficiency syndrome. Free Radical Biol. Med. 33, 192–200. - PubMed
-
- Marnett L. J. (1999) Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 424, 83–95. - PubMed
-
- Dedon P. C. (2008) The chemical toxicology of 2-deoxyribose oxidation in DNA. Chem. Res. Toxicol. 21, 206–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

