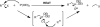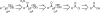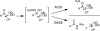Room-temperature alternative to the Arbuzov reaction: the reductive deoxygenation of acyl phosphonates
- PMID: 20726566
- PMCID: PMC2941390
- DOI: 10.1021/ol1015493
Room-temperature alternative to the Arbuzov reaction: the reductive deoxygenation of acyl phosphonates
Abstract
The reductive deoxygenation of acyl phosphonates using a Wolff-Kishner-like sequence is described. This transformation allows direct access to alkyl phosphonates from acyl phosphonates at room temperature. The method can be combined with acyl phosphonate synthesis into a one pot, four-step procedure for the conversion of carboxylic acids into alkyl phosphonates. The methodology works well for a variety of aliphatic acids and shows a functional group tolerance similar to that of other hydrazone-forming reactions.
Figures
Similar articles
-
Diametric stereocontrol in dynamic catalytic reduction of racemic acyl phosphonates: divergence from α-keto ester congeners.J Am Chem Soc. 2013 Jan 16;135(2):594-7. doi: 10.1021/ja310980q. Epub 2013 Jan 8. J Am Chem Soc. 2013. PMID: 23297694 Free PMC article.
-
Addition of trifluoromethyltrimethylsilane to acyl phosphonates: synthesis of TMS-protected 1-alkyl-1-trifluoromethyl-1-hydroxyphosphonates and 1-aryldifluoroethenyl phosphates.J Org Chem. 2007 Oct 26;72(22):8527-30. doi: 10.1021/jo070913c. Epub 2007 Oct 2. J Org Chem. 2007. PMID: 17910497
-
Direct transformation of amides into α-amino phosphonates via a reductive phosphination process.Org Lett. 2013 Aug 16;15(16):4214-7. doi: 10.1021/ol4019419. Epub 2013 Aug 2. Org Lett. 2013. PMID: 23909613
-
Recent advances in the synthesis of cyclic 5'-nornucleoside phosphonate analogues.Carbohydr Res. 2018 Jun 30;463:47-106. doi: 10.1016/j.carres.2018.04.009. Epub 2018 Apr 28. Carbohydr Res. 2018. PMID: 29772449 Review.
-
Recent Advances in the Synthesis of 5'-deoxynucleoside Phosphonate Analogs.Curr Med Chem. 2022;29(22):3857-3921. doi: 10.2174/0929867328666211111162447. Curr Med Chem. 2022. PMID: 34766884 Review.
Cited by
-
Optimization and a Kinetic Study on the Acidic Hydrolysis of Dialkyl α-Hydroxybenzylphosphonates.Molecules. 2020 Aug 20;25(17):3793. doi: 10.3390/molecules25173793. Molecules. 2020. PMID: 32825450 Free PMC article.
-
Improvements, Variations and Biomedical Applications of the Michaelis-Arbuzov Reaction.Int J Mol Sci. 2022 Mar 21;23(6):3395. doi: 10.3390/ijms23063395. Int J Mol Sci. 2022. PMID: 35328816 Free PMC article. Review.
-
Mechanically induced solvent-free esterification method at room temperature.RSC Adv. 2021 Jan 28;11(9):5080-5085. doi: 10.1039/d0ra09437d. eCollection 2021 Jan 25. RSC Adv. 2021. PMID: 35424454 Free PMC article.
References
-
- Engel R, Cohen JI. Synthesis of carbon-phosphorus bonds. 2nd ed. CRC Press; Boca Raton, FL: 2004.
- Savignac P, Iorga B. Modern phosphonate chemistry. CRC Press; Boca Raton, FL: 2003.
-
- Boutagy J, Thomas R. Chem. Rev. 1974;74:87.
- Horner L, Hoffmann H, Wippel HG. Chem. Ber.-Recl. 1958;91:61.
- Horner L, Hoffmann H, Wippel HG, Klahre G. Chem. Ber.-Recl. 1959;92:2499.
- Wadsworth W, Emmons WD. J. Am. Chem. Soc. 1961;83:1733.
-
- De Clercq E. Nat. Rev. Drug Discov. 2002;1:13. - PubMed
- De Clercq E, Holy A. Nat. Rev. Drug Discov. 2005;4:928. - PubMed
- Metcalf WW, van der Donk WA. Ann. Rev. Biochem. 2009;78:65. - PMC - PubMed
- Okuhara M, Goto T. Drugs Exp. Clin. Res. 1981;7:559.
- Okuhara M, Kuroda Y, Goto T, Okamoto M, Terano H, Kohsaka M, Aoki H, Imanaka H. J. Antibiot. 1980;33:13. - PubMed
- White AK, Metcalf WW. Ann. Rev. Microbiol. 2007;61:379. - PubMed
-
- Brandt GS. Ph.D. Dissertation. California Institute of Technology; Pasadena, CA: 2003.
- Chen L, Wu L, Otaka A, Smyth MS, Roller PP, Burke TR, Denhertog J, Zhang ZY. Biochem. Biophys. Res. Commun. 1995;216:976. - PubMed
- Engel R. Chem. Rev. 1977;77:349.
- Nieschalk J, Ohagan D. J. Chem. Soc., Chem. Commun. 1995:719.
- Oleksyszyn J, Powers JC. Proteolytic Enzymes: Serine and Cysteine Peptidases. Vol. 244. Academic Press Inc; San Diego: 1994. p. 423.
- Panigrahi K, Eggen M, Maeng JH, Shen QR, Berkowitz DB. Chem. Biol. 2009;16:928. - PMC - PubMed
- Petersson EJ. Ph.D Dissertation. California Institute of Technology; Pasadena, CA: 2005.
- Rothman DM, Petersson EJ, Vazquez ME, Brandt GS, Dougherty DA, Imperiali B. J. Am Chem Soc. 2005;127:846. - PubMed
- Zheng WP, Schwarzer D, LeBeau A, Weller JL, Klein DC, Cole PA. J. Biol. Chem. 2005;280:10462. - PubMed
- Zheng WP, Zhang ZS, Ganguly S, Weller JL, Klein DC, Cole PA. Nat. Struct. Biol. 2003;10:1054. - PubMed
-
-
Also known as the Michaelis-Arbuzov reaction.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources