CO and O2 binding to pseudo-tetradentate ligand-copper(I) complexes with a variable N-donor moiety: kinetic/thermodynamic investigation reveals ligand-induced changes in reaction mechanism
- PMID: 20726586
- PMCID: PMC2952189
- DOI: 10.1021/ja104107q
CO and O2 binding to pseudo-tetradentate ligand-copper(I) complexes with a variable N-donor moiety: kinetic/thermodynamic investigation reveals ligand-induced changes in reaction mechanism
Abstract
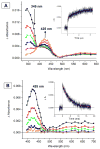
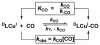
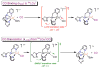
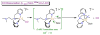
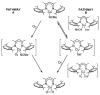
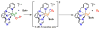
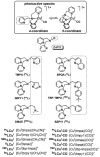
The kinetics, thermodynamics, and coordination dynamics are reported for O(2) and CO 1:1 binding to a series of pseudo-tetradentate ligand-copper(I) complexes ((D)LCu(I)) to give Cu(I)/O(2) and Cu(I)/CO product species. Members of the (D)LCu(I) series possess an identical tridentate core structure where the cuprous ion binds to the bispicolylamine (L) fragment. (D)L also contains a fourth variable N-donor moiety {D = benzyl (Bz); pyridyl (Py); imidazolyl (Im); dimethylamino (NMe(2)); (tert-butylphenyl)pyridyl (TBP); quinolyl (Q)}. The structural characteristics of (D)LCu(I)-CO and (D)LCu(I) are detailed, with X-ray crystal structures reported for (TBP)LCu(I)-CO, (Bz)LCu(I)-CO, and (Q)LCu(I). Infrared studies (solution and solid-state) confirm that (D)LCu(I)-CO possess the same four-coordinate core structure in solution with the variable D moiety "dangling", i.e., not coordinated to the copper(I) ion. Other trends observed for the present series appear to derive from the degree to which the D-group interacts with the cuprous ion center. Electrochemical studies reveal close similarities of behavior for (Im)LCu(I) and (NMe(2))LCu(I) (as well as for (TBP)LCu(I) and (Q)LCu(I)), which relate to the O(2) binding kinetics and thermodynamics. Equilibrium CO binding data (K(CO), ΔH°, ΔS°) were obtained by conducting UV-visible spectrophotometric CO titrations, while CO binding kinetics and thermodynamics (k(CO), ΔH(double dagger), ΔS(double dagger)) were measured through variable-temperature (193-293 K) transient absorbance laser flash photolysis experiments, λ(ex) = 355 nm. Carbon monoxide dissociation rate constants (k(-CO)) and corresponding activation parameters (ΔH(double dagger), ΔS(double dagger)) have also been obtained. CO binding to (D)LCu(I) follows an associative mechanism, with the increased donation from D leading to higher k(CO) values. Unlike observations from previous work, the K(CO) values increased as the k(CO) and k(-CO) values declined; the latter decreased at a faster rate. By using the "flash-and-trap" method (λ(ex) = 355 nm, 188-218 K), the kinetics and thermodynamics (k(O(2)), ΔH(double dagger), ΔS(double dagger)) for O(2) binding to (NMe(2))LCu(I) and (Im)LCu(I) were measured and compared to those for (Py)LCu(I). A surprising change in the O(2) binding mechanism was deduced from the thermodynamic ΔS(double dagger) values observed, associative for (Py)LCu(I) but dissociative for (NMe(2))LCu(I) and (Im)LCu(I); these results are interpreted as arising from a difference in the timing of electron transfer from copper(I) to O(2) as this molecule coordinates and a tetrahydrofuran (THF) solvent molecule dissociates. The change in mechanism was not simply related to alterations in (D)LCu(II/I) geometries or the order in which O(2) and THF coordinate. The equilibrium O(2) binding constant (K(O(2)), ΔH°, ΔS°) and O(2) dissociation rate constants (k(-O(2)), ΔH(double dagger), ΔS(double dagger)) were also determined. Overall the results demonstrate that subtle changes in the coordination environment, as occur over time through evolution in nature or through controlled ligand design in synthetic systems, dictate to a critically detailed level the observed chemistry in terms of reaction kinetics, structure, and reactivity, and thus function. Results reported here are also compared to relevant copper and/or iron biological systems and analogous synthetic ligand-copper systems.
Figures

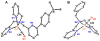





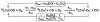

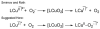
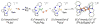
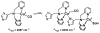
Similar articles
-
Copper(I)-dioxygen reactivity of [(L)Cu(I)](+) (L = tris(2-pyridylmethyl)amine): kinetic/thermodynamic and spectroscopic studies concerning the formation of Cu-O2 and Cu2-O2 adducts as a function of solvent medium and 4-pyridyl ligand substituent variations.Inorg Chem. 2003 Mar 24;42(6):1807-24. doi: 10.1021/ic0205684. Inorg Chem. 2003. PMID: 12639113
-
Carbon monoxide coordination and reversible photodissociation in copper(I) pyridylalkylamine compounds.Inorg Chem. 2008 Jan 7;47(1):241-56. doi: 10.1021/ic701903h. Epub 2007 Dec 4. Inorg Chem. 2008. PMID: 18052158
-
The rate of O2 and CO binding to a copper complex, determined by a "flash-and-trap" technique, exceeds that for hemes.J Am Chem Soc. 2003 Oct 1;125(39):11866-71. doi: 10.1021/ja034911v. J Am Chem Soc. 2003. PMID: 14505408
-
Copper(I)/O2 chemistry with imidazole containing tripodal tetradentate ligands leading to mu-1,2-peroxo-dicopper(II) species.Inorg Chem. 2009 Dec 7;48(23):11297-309. doi: 10.1021/ic9017695. Inorg Chem. 2009. PMID: 19886646 Free PMC article.
-
[CuO](+) and [CuOH](2+) complexes: intermediates in oxidation catalysis?Acc Chem Res. 2015 Jul 21;48(7):2126-31. doi: 10.1021/acs.accounts.5b00169. Epub 2015 Jun 15. Acc Chem Res. 2015. PMID: 26075312 Free PMC article. Review.
Cited by
-
A Four-Coordinate End-On Superoxocopper(II) Complex: Probing the Link between Coordination Number and Reactivity.J Am Chem Soc. 2024 Aug 28;146(34):23704-23716. doi: 10.1021/jacs.3c12268. Epub 2024 Aug 14. J Am Chem Soc. 2024. PMID: 39192778 Free PMC article.
-
Kinetics and thermodynamics of formation and electron-transfer reactions of Cu-O2 and Cu2-O2 complexes.Coord Chem Rev. 2013 Jan 1;257(1):187-195. doi: 10.1016/j.ccr.2012.05.031. Epub 2012 Jun 1. Coord Chem Rev. 2013. PMID: 23470920 Free PMC article.
-
Excitation wavelength dependent O2 release from copper(II)-superoxide compounds: laser flash-photolysis experiments and theoretical studies.J Am Chem Soc. 2014 Jan 29;136(4):1260-3. doi: 10.1021/ja4115314. Epub 2014 Jan 15. J Am Chem Soc. 2014. PMID: 24428309 Free PMC article.
-
Revisiting Oxygen Transport Features of Hemocyanin with NEVPT2 Level QM/MM Calculations.J Chem Theory Comput. 2025 Feb 25;21(4):2108-2117. doi: 10.1021/acs.jctc.4c01668. Epub 2025 Feb 6. J Chem Theory Comput. 2025. PMID: 39913244 Free PMC article.
-
Amine oxidative N-dealkylation via cupric hydroperoxide Cu-OOH homolytic cleavage followed by site-specific fenton chemistry.J Am Chem Soc. 2015 Mar 4;137(8):2867-74. doi: 10.1021/ja508371q. Epub 2015 Feb 23. J Am Chem Soc. 2015. PMID: 25706825 Free PMC article.
References
-
- Kim E, Chufan EE, Kamaraj K, Karlin KD. chem Rev. 2004;104:1077–1133. - PubMed
-
- Wasser IM, de Vries S, Moenne-Loccoz P, Schroder I, Karlin KD. Chem Rev. 2002;102:1201–1234. - PubMed
-
- Lucas HR, Karlin KD. Met Ions Life Sci. 2009;6:295–362. - PubMed
-
- Lewis EA, Tolman WB. Chem Rev. 2004;104:1047–1076. - PubMed
-
- Mirica LM, Ottenwaelder X, Stack TDP. Chem Rev. 2004;104:1013–1045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

