Survival of hepatitis C virus in syringes: implication for transmission among injection drug users
- PMID: 20726768
- PMCID: PMC2932767
- DOI: 10.1086/656212
Survival of hepatitis C virus in syringes: implication for transmission among injection drug users
Abstract
Background: We hypothesized that the high prevalence of hepatitis C virus (HCV) among injection drug users might be due to prolonged virus survival in contaminated syringes.
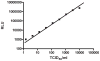
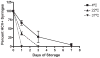
Methods: We developed a microculture assay to examine the viability of HCV. Syringes were loaded with blood spiked with HCV reporter virus (Jc1/GLuc2A) to simulate 2 scenarios of residual volumes: low void volume (2 microL) for 1-mL insulin syringes and high void volume (32 microL) for 1-mL tuberculin syringes. Syringes were stored at 4 degrees C, 22 degrees C, and 37 degrees C for up to 63 days before testing for HCV infectivity by using luciferase activity.
Results: The virus decay rate was biphasic (t1/2alpha= 0.4 h and t1/2beta = 28 hh). Insulin syringes failed to yield viable HCV beyond day 1 at all storage temperatures except 4 degrees , in which 5% of syringes yielded viable virus on day 7. Tuberculin syringes yielded viable virus from 96%, 71%, and 52% of syringes after storage at 4 degrees, 22 degrees, and 37 degrees for 7 days, respectively, and yielded viable virus up to day 63.
Conclusions: The high prevalence of HCV among injection drug users may be partly due to the resilience of the virus and the syringe type. Our findings may be used to guide prevention strategies.
Conflict of interest statement
The authors do not have a commercial or other association that might pose a conflict of interest.
Figures


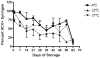
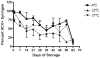
Comment in
-
The beginning of a new era in understanding hepatitis C virus prevention.J Infect Dis. 2010 Oct 1;202(7):981-3. doi: 10.1086/656213. J Infect Dis. 2010. PMID: 20726769 Free PMC article.
-
Thermodynamic analysis of hepatitis C virus vitality in syringes.J Infect Dis. 2011 Jun 1;203(11):1696-7. doi: 10.1093/infdis/jir143. Epub 2011 May 5. J Infect Dis. 2011. PMID: 21550996 No abstract available.
References
-
- Cohen J. The scientific challenge of hepatitis C. Science. 1999;285:26–30. - PubMed
-
- Shustov AV, Kochneva GV, Sivolobova GF, et al. Molecular epidemiology of the hepatitis C virus in Western Siberia. J Med Virol. 2005;77:382–9. - PubMed
-
- Ruzibakiev R, Kato H, Ueda R, et al. Risk factors and seroprevalence of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus infection in uzbekistan. Intervirology. 2001;44:327–32. - PubMed
-
- Onishchenko GG, Shakhgil'dian IV. The current problems in the epidemiology and prevention of viral hepatitis B and C in the Russian Federation. Zh Mikrobiol Epidemiol Immunobiol. 2000:50–4. - PubMed

