Inhibition of apoptosis by progesterone in cardiomyocytes
- PMID: 20726854
- PMCID: PMC4133411
- DOI: 10.1111/j.1474-9726.2010.00619.x
Inhibition of apoptosis by progesterone in cardiomyocytes
Abstract
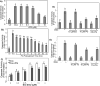
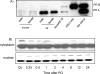
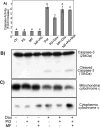
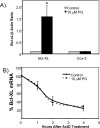
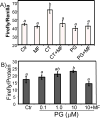
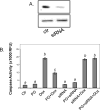
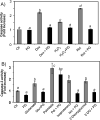
While gender-based differences in heart disease have raised the possibility that estrogen (ES) or progesterone (PG) may have cardioprotective effects, recent controversy regarding hormone replacement therapy has questioned the cardiac effects of these steroids. Using cardiomyocytes, we tested whether ES or PG has protective effects at the cellular level. We found that PG but not ES protects cardiomyocytes from apoptotic cell death induced by doxorubicin (Dox). PG inhibited apoptosis in a dose-dependent manner, by 12 ± 4.0% at 1 μm and 60 ± 1.0% at 10 μm. The anti-apoptotic effect of PG was also time dependent, causing 18 ± 5% or 62 + 2% decrease in caspase-3 activity within 1 h or 72 h of pretreatment. While PG causes nuclear translocation of its receptor within 20 min, the cytoprotective effect of PG was canceled by mifepristone (MF), a PG receptor antagonist. Analyses using Affymetrix high-density oligonucleotide array and RT-PCR found that PG induced Bcl-xL, metallothionine, NADPH quinone oxidoreductase 1, glutathione peroxidase-3, and four isoforms of glutathione S-transferase. Western blot analyses revealed that PG indeed induced an elevation of Bcl-xL protein in a dose- and time-dependent manner. Nuclear run-on assay indicated that PG induced Bcl-xL gene transcription. Inhibiting the expression of Bcl-xL using siRNA reduced the cytoprotective effect of PG. Our data suggests that PG induces a cytoprotective effect in cardiomyocytes in association with induction of Bcl-xL gene.
© 2010 The Authors Aging Cell © 2010 Blackwell Publishing Ltd/Anatomical Society of Great Britain and Ireland.
Figures



Similar articles
-
Differential Regulation of Bcl-xL Gene Expression by Corticosterone, Progesterone, and Retinoic Acid.J Biochem Mol Toxicol. 2016 Jun;30(6):309-16. doi: 10.1002/jbt.21795. Epub 2016 Feb 25. J Biochem Mol Toxicol. 2016. PMID: 26915917 Free PMC article.
-
Corticosteroids inhibit cell death induced by doxorubicin in cardiomyocytes: induction of antiapoptosis, antioxidant, and detoxification genes.Mol Pharmacol. 2005 Jun;67(6):1861-73. doi: 10.1124/mol.104.003814. Epub 2005 Mar 8. Mol Pharmacol. 2005. PMID: 15755911
-
Glucocorticoid induced leucine zipper inhibits apoptosis of cardiomyocytes by doxorubicin.Toxicol Appl Pharmacol. 2014 Apr 1;276(1):55-62. doi: 10.1016/j.taap.2014.01.013. Epub 2014 Jan 28. Toxicol Appl Pharmacol. 2014. PMID: 24480152 Free PMC article.
-
Glycoprotein 130 regulates cardiac myocyte survival in doxorubicin-induced apoptosis through phosphatidylinositol 3-kinase/Akt phosphorylation and Bcl-xL/caspase-3 interaction.Circulation. 2001 Jan 30;103(4):555-61. doi: 10.1161/01.cir.103.4.555. Circulation. 2001. PMID: 11157722
-
Hsp10 and Hsp60 modulate Bcl-2 family and mitochondria apoptosis signaling induced by doxorubicin in cardiac muscle cells.J Mol Cell Cardiol. 2003 Sep;35(9):1135-43. doi: 10.1016/s0022-2828(03)00229-3. J Mol Cell Cardiol. 2003. PMID: 12967636
Cited by
-
Carbamylated erythropoietin attenuates cardiomyopathy via PI3K/Akt activation in rats with diabetic cardiomyopathy.Exp Ther Med. 2013 Aug;6(2):567-573. doi: 10.3892/etm.2013.1134. Epub 2013 May 31. Exp Ther Med. 2013. PMID: 24137228 Free PMC article.
-
PDK4 Inhibits Cardiac Pyruvate Oxidation in Late Pregnancy.Circ Res. 2017 Dec 8;121(12):1370-1378. doi: 10.1161/CIRCRESAHA.117.311456. Epub 2017 Sep 19. Circ Res. 2017. PMID: 28928113 Free PMC article.
-
Correlation between steroid hormonal levels and cardiac function in women during controlled ovarian hyperstimulation.Endocrine. 2013 Dec;44(3):784-9. doi: 10.1007/s12020-013-9953-7. Epub 2013 Apr 11. Endocrine. 2013. PMID: 23576024
-
A luminance-based heart chip assay for assessing the efficacy of graft preservation solutions in heart transplantation in rats.Heart Asia. 2013 Jan 17;5(1):7-14. doi: 10.1136/heartasia-2012-010160. Print 2013. Heart Asia. 2013. PMID: 23585802 Free PMC article.
-
Does Birth Trigger Cell Death in the Developing Brain?eNeuro. 2020 Feb 14;7(1):ENEURO.0517-19.2020. doi: 10.1523/ENEURO.0517-19.2020. Print 2020 Jan/Feb. eNeuro. 2020. PMID: 32015098 Free PMC article.
References
-
- Anversa P, Olivetti G, Leri A, Liu Y, Kajstura J. Myocyte cell death and ventricular remodeling. Curr Opin Nephrol Hypertens. 1997;6:169–176. - PubMed
-
- Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789–1792. - PubMed
-
- Berg MN, Dharmarajan AM, Waddell BJ. Glucocorticoids and progesterone prevent apoptosis in the lactating rat mammary gland. Endocrinology. 2002;143:222–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

