Spliceostatin A blocks angiogenesis by inhibiting global gene expression including VEGF
- PMID: 20726856
- PMCID: PMC11158552
- DOI: 10.1111/j.1349-7006.2010.01686.x
Spliceostatin A blocks angiogenesis by inhibiting global gene expression including VEGF
Abstract
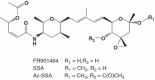
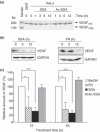
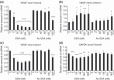
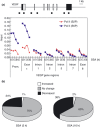
Spliceostatin A (SSA) is a methylated derivative of an antitumor natural product FR901464, which specifically binds and inhibits the SF3b spliceosome sub-complex. To investigate the selective antitumor activity of SSA, we focused on the regulation of vascular endothelial growth factor (VEGF) mRNA, since VEGF is a key regulatory component in tumor angiogenesis and known for the intricate regulation of mRNA processing, such as alternative splicing. We found that in HeLa cells SSA reduced the amount of both mRNA and protein of VEGF. Spliceostatin A not only inhibited the splicing reaction of VEGF pre-mRNA but also reduced the total amount of VEGF's transcripts, while SSA affected GAPDH mRNA to a lesser extent. Given a significant reduction in VEGF gene expression, SSA was expected to possess anti-angiogenic activity in vivo. Indeed, SSA inhibited cancer cell-derived angiogenesis in vivo in a chicken chorioallantoic membrane (CAM) assay. The inhibition of angiogenesis with SSA was abolished by addition of exogenous VEGF. We also performed global gene expression analyses of HeLa cells and found that the expression levels of 38% of total genes including VEGF decreased to <50% of the basal levels following 16 h of SSA treatment. These results suggest that the global interference of gene expression including VEGF in tumor cells is at least one of the mechanisms by which SSA (or FR901464) exhibits its strong antitumor activity.
© 2010 Japanese Cancer Association.
Figures


Similar articles
-
Global analysis of pre-mRNA subcellular localization following splicing inhibition by spliceostatin A.RNA. 2017 Jan;23(1):47-57. doi: 10.1261/rna.058065.116. Epub 2016 Oct 17. RNA. 2017. PMID: 27754875 Free PMC article.
-
Antiangiogenic Potential of Microbial Metabolite Elaiophylin for Targeting Tumor Angiogenesis.Molecules. 2018 Mar 2;23(3):563. doi: 10.3390/molecules23030563. Molecules. 2018. PMID: 29498688 Free PMC article.
-
Fluocinolone inhibits VEGF expression via glucocorticoid receptor in human retinal pigment epithelial (ARPE-19) cells and TNF-alpha-induced angiogenesis in chick chorioallantoic membrane (CAM).J Ocul Pharmacol Ther. 2009 Apr;25(2):97-103. doi: 10.1089/jop.2008.0090. J Ocul Pharmacol Ther. 2009. PMID: 19284324
-
Norcantharidin: a potential antiangiogenic agent for gallbladder cancers in vitro and in vivo.Int J Oncol. 2012 May;40(5):1501-14. doi: 10.3892/ijo.2011.1314. Epub 2011 Dec 21. Int J Oncol. 2012. PMID: 22200632
-
The epigallocatechin gallate derivative Y6 inhibits human hepatocellular carcinoma by inhibiting angiogenesis in MAPK/ERK1/2 and PI3K/AKT/ HIF-1α/VEGF dependent pathways.J Ethnopharmacol. 2020 Sep 15;259:112852. doi: 10.1016/j.jep.2020.112852. Epub 2020 Apr 9. J Ethnopharmacol. 2020. PMID: 32278759
Cited by
-
Therapeutic Targeting of Alternative Splicing: A New Frontier in Cancer Treatment.Front Oncol. 2022 Apr 8;12:868664. doi: 10.3389/fonc.2022.868664. eCollection 2022. Front Oncol. 2022. PMID: 35463320 Free PMC article. Review.
-
Misregulation of pre-mRNA alternative splicing in cancer.Cancer Discov. 2013 Nov;3(11):1228-37. doi: 10.1158/2159-8290.CD-13-0253. Epub 2013 Oct 21. Cancer Discov. 2013. PMID: 24145039 Free PMC article. Review.
-
Splicing modulators: on the way from nature to clinic.J Antibiot (Tokyo). 2021 Oct;74(10):603-616. doi: 10.1038/s41429-021-00450-1. Epub 2021 Aug 3. J Antibiot (Tokyo). 2021. PMID: 34345042 Free PMC article. Review.
-
mRNA transcript diversity creates new opportunities for pharmacological intervention.Mol Pharmacol. 2012 May;81(5):620-30. doi: 10.1124/mol.111.076604. Epub 2012 Feb 7. Mol Pharmacol. 2012. PMID: 22319206 Free PMC article. Review.
-
The SF3B1 inhibitor spliceostatin A (SSA) elicits apoptosis in chronic lymphocytic leukaemia cells through downregulation of Mcl-1.Leukemia. 2016 Feb;30(2):351-60. doi: 10.1038/leu.2015.286. Epub 2015 Oct 21. Leukemia. 2016. PMID: 26488112
References
-
- Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti‐VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3(5): 391–400. - PubMed
-
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000; 407(6801): 249–57. - PubMed
-
- Matsumoto T, Claesson‐Welsh L. VEGF receptor signal transduction. Sci STKE 2001; 2001(112): RE21. - PubMed
-
- Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 2009; 29(6): 789–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

