Neogenesis and development of the high endothelial venules that mediate lymphocyte trafficking
- PMID: 20726857
- PMCID: PMC11158135
- DOI: 10.1111/j.1349-7006.2010.01687.x
Neogenesis and development of the high endothelial venules that mediate lymphocyte trafficking
Abstract
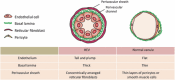
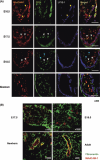
Physiological recruitment of lymphocytes from the blood into lymph nodes and Peyer's patches is mediated by high endothelial venules (HEV), specialized blood vessels found in secondary lymphoid tissues except for the spleen. The HEV are distinguished from other types of blood vessels by their tall and plump endothelial cells, and by their expression of specific chemokines and adhesion molecules, which all contribute to the selective lymphocyte trafficking across these blood vessels. The development of HEV is ontogenically regulated, and they appear perinatally in the mouse. High endothelial venules can appear ectopically, for instance in chronically inflamed tissues. Given that HEV enable the efficient trafficking of lymphocytes into tissues, the induction of HEV at a tumor site could potentiate tumor-specific immune responses, and the artificial manipulation of HEV neogenesis might thus provide a new tool for cancer immunotherapy. However, the process of HEV development and the mechanisms by which the unique features of HEV are maintained are incompletely understood. In this review, we discuss the process of HEV neogenesis and development during ontogeny, and their molecular requirements for maintaining their unique characteristics under physiological conditions.
© 2010 Japanese Cancer Association.
Figures

References
-
- Von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol 2003; 3: 867–78. - PubMed
-
- Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 2004; 4: 360–70. - PubMed
-
- Mebius RE, Dowbenko D, Williams A, Fennie C, Lasky LA, Watson SR. Expression of GlyCAM‐1, an endothelial ligand for L‐selectin, is affected by afferent lymphatic flow. J Immunol 1993; 151: 6769–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

