The microRNA pathway and cancer
- PMID: 20726859
- PMCID: PMC11159795
- DOI: 10.1111/j.1349-7006.2010.01683.x
The microRNA pathway and cancer
Abstract
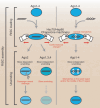
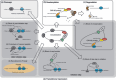
MicroRNAs (miRNAs) are ∼22nt long, non-coding RNAs that guide post-transcriptional gene silencing of their target genes and regulate diverse biological processes including cancer. miRNAs do not act alone, but require assembly into RNA-induced silencing complex (RISC). In this review, we summarize how miRNAs are produced, assembled into RISC, and regulate target mRNAs, and discuss how the miRNA pathway is involved in cancer.
© 2010 Japanese Cancer Association.
Figures


Similar articles
-
Intracellular and extracellular microRNA: An update on localization and biological role.Prog Histochem Cytochem. 2016 Nov;51(3-4):33-49. doi: 10.1016/j.proghi.2016.06.001. Epub 2016 Jun 25. Prog Histochem Cytochem. 2016. PMID: 27396686 Review.
-
Nuclear functions of mammalian MicroRNAs in gene regulation, immunity and cancer.Mol Cancer. 2018 Feb 22;17(1):64. doi: 10.1186/s12943-018-0765-5. Mol Cancer. 2018. PMID: 29471827 Free PMC article. Review.
-
MicroRNA Expression: Protein Participants in MicroRNA Regulation.Methods Mol Biol. 2017;1617:27-37. doi: 10.1007/978-1-4939-7046-9_2. Methods Mol Biol. 2017. PMID: 28540674 Review.
-
MicroRNAs: molecular features and role in cancer.Front Biosci (Landmark Ed). 2012 Jun 1;17(7):2508-40. doi: 10.2741/4068. Front Biosci (Landmark Ed). 2012. PMID: 22652795 Free PMC article. Review.
-
Target binding triggers hierarchical phosphorylation of human Argonaute-2 to promote target release.Elife. 2022 May 31;11:e76908. doi: 10.7554/eLife.76908. Elife. 2022. PMID: 35638597 Free PMC article.
Cited by
-
MicroRNA-regulated protein-protein interaction networks and their functions in breast cancer.Int J Mol Sci. 2013 May 30;14(6):11560-606. doi: 10.3390/ijms140611560. Int J Mol Sci. 2013. PMID: 23722663 Free PMC article.
-
Expression and clinical significance of microRNA-1246 in human oral squamous cell carcinoma.Med Sci Monit. 2015 Mar 16;21:776-81. doi: 10.12659/MSM.892508. Med Sci Monit. 2015. PMID: 25791131 Free PMC article.
-
The Expanding Role of Cancer Stem Cell Marker ALDH1A3 in Cancer and Beyond.Cancers (Basel). 2023 Jan 13;15(2):492. doi: 10.3390/cancers15020492. Cancers (Basel). 2023. PMID: 36672441 Free PMC article. Review.
-
Comprehensive Review of Genetic Association Studies and Meta-Analysis on polymorphisms in microRNAs and Urological Neoplasms Risk.Sci Rep. 2018 Feb 28;8(1):3776. doi: 10.1038/s41598-018-21749-4. Sci Rep. 2018. PMID: 29491365 Free PMC article. Review.
-
The N domain of Argonaute drives duplex unwinding during RISC assembly.Nat Struct Mol Biol. 2012 Jan 10;19(2):145-51. doi: 10.1038/nsmb.2232. Nat Struct Mol Biol. 2012. PMID: 22233755
References
-
- Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol 2006; 13: 1097–101. - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004; 116: 281–97. - PubMed
-
- Lee Y, Ahn C, Han J et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003; 425: 415–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

