Src family kinases mediate the inhibition of substance P release in the rat spinal cord by μ-opioid receptors and GABA(B) receptors, but not α2 adrenergic receptors
- PMID: 20726886
- PMCID: PMC2942982
- DOI: 10.1111/j.1460-9568.2010.07335.x
Src family kinases mediate the inhibition of substance P release in the rat spinal cord by μ-opioid receptors and GABA(B) receptors, but not α2 adrenergic receptors
Abstract
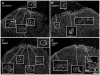
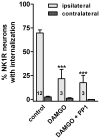
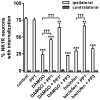
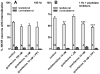
GABA(B) , μ-opioid and adrenergic α(2) receptors inhibit substance P release from primary afferent terminals in the dorsal horn. Studies in cell expression systems suggest that μ-opioid and GABA(B) receptors inhibit transmitter release from primary afferents by activating Src family kinases (SFKs), which then phosphorylate and inhibit voltage-gated calcium channels. This study investigated whether SFKs mediate the inhibition of substance P release by these three receptors. Substance P release was measured as neurokinin 1 receptor (NK1R) internalization in spinal cord slices and in vivo. In slices, NK1R internalization induced by high-frequency dorsal root stimulation was inhibited by the μ-opioid agonist DAMGO and the GABA(B) agonist baclofen. This inhibition was reversed by the SFK inhibitor PP1. NK1R internalization induced by low-frequency stimulation was also inhibited by DAMGO, but PP1 did not reverse this effect. In vivo, NK1R internalization induced by noxious mechanical stimulation of the hind paw was inhibited by intrathecal DAMGO and baclofen. This inhibition was reversed by intrathecal PP1, but not by the inactive PP1 analog PP3. PP1 produced no effect by itself. The α(2) adrenergic agonists medetomidine and guanfacine produced a small but statistically significant inhibition of NK1R internalization induced by low-frequency dorsal root stimulation. PP1 did not reverse the inhibition by guanfacine. These results show that SFKs mediate the inhibition of substance P release by μ-opioid and GABA(B) receptors, but not by α(2) receptors, which is probably mediated by the binding of G protein βγ subunits to calcium channels.
European Journal of Neuroscience © 2010 Federation of European Neuroscience Societies and Blackwell Publishing Ltd. No claim to original US government works.
Figures


Similar articles
-
μ-Opioid receptor inhibition of substance P release from primary afferents disappears in neuropathic pain but not inflammatory pain.Neuroscience. 2014 May 16;267:67-82. doi: 10.1016/j.neuroscience.2014.02.023. Epub 2014 Feb 26. Neuroscience. 2014. PMID: 24583035 Free PMC article.
-
Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release.J Neurosci. 2005 Apr 6;25(14):3651-60. doi: 10.1523/JNEUROSCI.0252-05.2005. J Neurosci. 2005. PMID: 15814796 Free PMC article.
-
Mechanisms of μ-opioid receptor inhibition of NMDA receptor-induced substance P release in the rat spinal cord.Neuropharmacology. 2018 Jan;128:255-268. doi: 10.1016/j.neuropharm.2017.10.014. Epub 2017 Oct 16. Neuropharmacology. 2018. PMID: 29042318 Free PMC article.
-
Cannabinoid CB1 receptor facilitation of substance P release in the rat spinal cord, measured as neurokinin 1 receptor internalization.Eur J Neurosci. 2010 Jan;31(2):225-37. doi: 10.1111/j.1460-9568.2009.07075.x. Epub 2010 Jan 13. Eur J Neurosci. 2010. PMID: 20074214 Free PMC article.
-
GABA(A) receptor facilitation of neurokinin release from primary afferent terminals in the rat spinal cord.Neuroscience. 2005;130(4):1013-27. doi: 10.1016/j.neuroscience.2004.10.019. Neuroscience. 2005. PMID: 15652997
Cited by
-
cAMP signaling through protein kinase A and Epac2 induces substance P release in the rat spinal cord.Neuropharmacology. 2021 May 15;189:108533. doi: 10.1016/j.neuropharm.2021.108533. Epub 2021 Mar 17. Neuropharmacology. 2021. PMID: 33744339 Free PMC article.
-
μ-Opioid receptor inhibition of substance P release from primary afferents disappears in neuropathic pain but not inflammatory pain.Neuroscience. 2014 May 16;267:67-82. doi: 10.1016/j.neuroscience.2014.02.023. Epub 2014 Feb 26. Neuroscience. 2014. PMID: 24583035 Free PMC article.
-
Mu-opioid receptors in nociceptive afferents produce a sustained suppression of hyperalgesia in chronic pain.Pain. 2018 Aug;159(8):1607-1620. doi: 10.1097/j.pain.0000000000001247. Pain. 2018. PMID: 29677019 Free PMC article.
-
BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals.Eur J Neurosci. 2014 May;39(9):1439-54. doi: 10.1111/ejn.12516. Epub 2014 Mar 11. Eur J Neurosci. 2014. PMID: 24611998 Free PMC article.
-
Endomorphin-2 Inhibition of Substance P Signaling within Lamina I of the Spinal Cord Is Impaired in Diabetic Neuropathic Pain Rats.Front Mol Neurosci. 2017 Jan 10;9:167. doi: 10.3389/fnmol.2016.00167. eCollection 2016. Front Mol Neurosci. 2017. PMID: 28119567 Free PMC article.
References
-
- Aimone LD, Jones SL, Gebhart GF. Stimulation-produced descending inhibition from the periaqueductal gray and nucleus raphe magnus in the rat: mediation by spinal monoamines but not opioids. Pain. 1987;31:123–136. - PubMed
-
- Allen BJ, Li J, Menning PM, Rogers SD, Ghilardi J, Mantyh PW, Simone DA. Primary afferent fibers that contribute to increased substance P receptor internalization in the spinal cord after injury. J Neurophysiol. 1999;81:1379–1390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

