β-Secretase-1 elevation in aged monkey and Alzheimer's disease human cerebral cortex occurs around the vasculature in partnership with multisystem axon terminal pathogenesis and β-amyloid accumulation
- PMID: 20726888
- PMCID: PMC2970759
- DOI: 10.1111/j.1460-9568.2010.07376.x
β-Secretase-1 elevation in aged monkey and Alzheimer's disease human cerebral cortex occurs around the vasculature in partnership with multisystem axon terminal pathogenesis and β-amyloid accumulation
Abstract
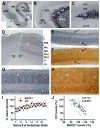
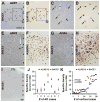
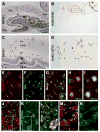
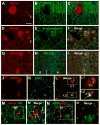
Alzheimer's disease (AD) is the most common dementia-causing disorder in the elderly; it may be related to multiple risk factors, and is characterized pathologically by cerebral hypometabolism, paravascular β-amyloid peptide (Aβ) plaques, neuritic dystrophy, and intra-neuronal aggregation of phosphorylated tau. To explore potential pathogenic links among some of these lesions, we examined β-secretase-1 (BACE1) alterations relative to Aβ deposition, neuritic pathology and vascular organization in aged monkey and AD human cerebral cortex. Western blot analyses detected increased levels of BACE1 protein and β-site-cleavage amyloid precursor protein C-terminal fragments in plaque-bearing human and monkey cortex relative to controls. In immunohistochemistry, locally elevated BACE1 immunoreactivity (IR) occurred in AD but not in control human cortex, with a trend for increased overall density among cases with greater plaque pathology. In double-labeling preparations, BACE1 IR colocalized with immunolabeling for Aβ but not for phosphorylated tau. In perfusion-fixed monkey cortex, locally increased BACE1 IR co-existed with intra-axonal and extracellular Aβ IR among virtually all neuritic plaques, ranging from primitive to typical cored forms. This BACE1 labeling localized to swollen/sprouting axon terminals that might co-express one or another neuronal phenotype markers (GABAergic, glutamatergic, cholinergic, or catecholaminergic). Importantly, these BACE1-labeled dystrophic axons resided near to or in direct contact with blood vessels. These findings suggest that plaque formation in AD or normal aged primates relates to a multisystem axonal pathogenesis that occurs in partnership with a potential vascular or metabolic deficit. The data provide a mechanistic explanation for why senile plaques are present preferentially near the cerebral vasculature.
© 2010 The Authors. European Journal of Neuroscience © 2010 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures



Similar articles
-
BACE1 elevation is involved in amyloid plaque development in the triple transgenic model of Alzheimer's disease: differential Aβ antibody labeling of early-onset axon terminal pathology.Neurotox Res. 2012 Feb;21(2):160-74. doi: 10.1007/s12640-011-9256-9. Epub 2011 Jul 2. Neurotox Res. 2012. PMID: 21725719 Free PMC article.
-
Beta-secretase-1 elevation in transgenic mouse models of Alzheimer's disease is associated with synaptic/axonal pathology and amyloidogenesis: implications for neuritic plaque development.Eur J Neurosci. 2009 Dec;30(12):2271-83. doi: 10.1111/j.1460-9568.2009.07017.x. Epub 2009 Dec 10. Eur J Neurosci. 2009. PMID: 20092570 Free PMC article.
-
Functional deprivation promotes amyloid plaque pathogenesis in Tg2576 mouse olfactory bulb and piriform cortex.Eur J Neurosci. 2010 Feb;31(4):710-21. doi: 10.1111/j.1460-9568.2010.07103.x. Eur J Neurosci. 2010. PMID: 20384814 Free PMC article.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Effects of altered RTN3 expression on BACE1 activity and Alzheimer's neuritic plaques.Rev Neurosci. 2017 Feb 1;28(2):145-154. doi: 10.1515/revneuro-2016-0054. Rev Neurosci. 2017. PMID: 27883331 Review.
Cited by
-
Transformation of non-neuritic into neuritic plaques during AD progression drives cortical spread of tau pathology via regenerative failure.Acta Neuropathol Commun. 2023 Dec 1;11(1):190. doi: 10.1186/s40478-023-01688-6. Acta Neuropathol Commun. 2023. PMID: 38037144 Free PMC article.
-
BACE1 elevation is involved in amyloid plaque development in the triple transgenic model of Alzheimer's disease: differential Aβ antibody labeling of early-onset axon terminal pathology.Neurotox Res. 2012 Feb;21(2):160-74. doi: 10.1007/s12640-011-9256-9. Epub 2011 Jul 2. Neurotox Res. 2012. PMID: 21725719 Free PMC article.
-
Distinct patterns of APP processing in the CNS in autosomal-dominant and sporadic Alzheimer disease.Acta Neuropathol. 2013 Feb;125(2):201-13. doi: 10.1007/s00401-012-1062-9. Epub 2012 Dec 6. Acta Neuropathol. 2013. PMID: 23224319 Free PMC article.
-
Experimental microembolism induces localized neuritic pathology in guinea pig cerebrum.Oncotarget. 2015 May 10;6(13):10772-85. doi: 10.18632/oncotarget.3599. Oncotarget. 2015. PMID: 25871402 Free PMC article.
-
γ-secretase binding sites in aged and Alzheimer's disease human cerebrum: the choroid plexus as a putative origin of CSF Aβ.Eur J Neurosci. 2013 May;37(10):1714-25. doi: 10.1111/ejn.12159. Epub 2013 Feb 22. Eur J Neurosci. 2013. PMID: 23432732 Free PMC article.
References
-
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. - PubMed
-
- Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, Wilkinson D, Holmes C, Nicoll JA. Consequence of Abeta immunization on the vasculature of human Alzheimer's disease brain. Brain. 2008;131:3299–3310. - PubMed
-
- Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson's disease? Neurobiol Dis. 2007;25:134–149. - PubMed
-
- Cohen AD, Price JC, Weissfeld LA, James J, Rosario BL, Bi W, Nebes RD, Saxton JA, Snitz BE, Aizenstein HA, Wolk DA, Dekosky ST, Mathis CA, Klunk WE. Basal cerebral metabolism may modulate the cognitive effects of Abeta in mild cognitive impairment: an example of brain reserve. J Neurosci. 2009;29:14770–14778. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

