Prion amyloid structure explains templating: how proteins can be genes
- PMID: 20726897
- PMCID: PMC3025496
- DOI: 10.1111/j.1567-1364.2010.00666.x
Prion amyloid structure explains templating: how proteins can be genes
Abstract
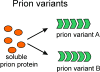
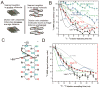
The yeast and fungal prions determine heritable and infectious traits, and are thus genes composed of protein. Most prions are inactive forms of a normal protein as it forms a self-propagating filamentous β-sheet-rich polymer structure called amyloid. Remarkably, a single prion protein sequence can form two or more faithfully inherited prion variants, in effect alleles of these genes. What protein structure explains this protein-based inheritance? Using solid-state nuclear magnetic resonance, we showed that the infectious amyloids of the prion domains of Ure2p, Sup35p and Rnq1p have an in-register parallel architecture. This structure explains how the amyloid filament ends can template the structure of a new protein as it joins the filament. The yeast prions [PSI(+)] and [URE3] are not found in wild strains, indicating that they are a disadvantage to the cell. Moreover, the prion domains of Ure2p and Sup35p have functions unrelated to prion formation, indicating that these domains are not present for the purpose of forming prions. Indeed, prion-forming ability is not conserved, even within Saccharomyces cerevisiae, suggesting that the rare formation of prions is a disease. The prion domain sequences generally vary more rapidly in evolution than does the remainder of the molecule, producing a barrier to prion transmission, perhaps selected in evolution by this protection.
Journal compilation © 2010 Federation of European Microbiological Societies. Published by Blackwell Publishing Ltd. No claim to original US government works.
Figures


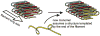
References
-
- Alper T, Haig DA, Clarke MC. The exceptionally small size of the scrapie agent. Biochem Biophys Res Commun. 1966;22:278 – 284. - PubMed
-
- Alper T, Cramp WA, Haig DA, Clarke MC. Does the agent of scrapie replicate without nucleic acid? Nature. 1967;214:764 – 766. - PubMed
-
- Bai M, Zhou JM, Perrett S. The yeast prion protein Ure2 shows glutathione peroxidase activity in both native and fibrillar forms. J Biol Chem. 2004;279:50025–50030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

