Inhibitors of catechol-O-methyltransferase sensitize mice to pain
- PMID: 20726980
- PMCID: PMC3010567
- DOI: 10.1111/j.1476-5381.2010.00999.x
Inhibitors of catechol-O-methyltransferase sensitize mice to pain
Abstract
Background and purpose: Catechol-O-methyltransferase (COMT) inhibitors are used in Parkinson's disease in which pain is an important symptom. COMT polymorphisms modulate pain and opioid analgesia in humans. In rats, COMT inhibitors have been shown to be pro-nociceptive in acute pain models, but also to attenuate allodynia and hyperalgesia in a model of diabetic neuropathy. Here, we have assessed the effects of acute and repeated administrations of COMT inhibitors on mechanical, thermal and carrageenan-induced nociception in male mice.
Experimental approach: We used single and repeated administration of a peripherally restricted, short-acting (nitecapone) and also a centrally acting (3,5-dinitrocatechol, OR-486) COMT inhibitor. We also tested CGP 28014, an indirect inhibitor of COMT enzyme. Effects of OR-486 on thermal nociception were also studied in COMT deficient mice. Effects on spinal pathways were assessed in rats given intrathecal nitecapone.
Key results: After single administration, both nitecapone and OR-486 reduced mechanical nociceptive thresholds and thermal nociceptive latencies (hot plate test) at 2 and 3 h, regardless of their brain penetration. These effects were still present after chronic treatment with COMT inhibitors for 5 days. Intraplantar injection of carrageenan reduced nociceptive latencies and both COMT inhibitors potentiated this reduction without modifying inflammation. CGP 28014 shortened paw flick latencies. OR-486 did not modify hot plate times in Comt gene deficient mice. Intrathecal nitecapone modified neither thermal nor mechanical nociception.
Conclusions and implications: Pro-nociceptive effects of COMT inhibitors were confirmed. The pro-nociceptive effects were primarily mediated via mechanisms acting outside the brain and spinal cord. COMT protein was required for these actions.
Figures
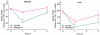
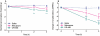
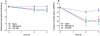
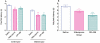
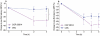
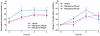
Similar articles
-
Nitecapone reduces development and symptoms of neuropathic pain after spinal nerve ligation in rats.Eur J Pain. 2011 Aug;15(7):732-40. doi: 10.1016/j.ejpain.2010.12.001. Epub 2011 Jan 8. Eur J Pain. 2011. PMID: 21216640
-
Pain behavior and response properties of spinal dorsal horn neurons following experimental diabetic neuropathy in the rat: modulation by nitecapone, a COMT inhibitor with antioxidant properties.Exp Neurol. 2001 Feb;167(2):425-34. doi: 10.1006/exnr.2000.7574. Exp Neurol. 2001. PMID: 11161631
-
Stress-induced analgesia and morphine responses are changed in catechol-O-methyltransferase-deficient male mice.Basic Clin Pharmacol Toxicol. 2008 Oct;103(4):367-73. doi: 10.1111/j.1742-7843.2008.00289.x. Basic Clin Pharmacol Toxicol. 2008. PMID: 18834357
-
Catechol-O-methyltransferase and pain.Int Rev Neurobiol. 2010;95:227-79. doi: 10.1016/B978-0-12-381326-8.00010-7. Int Rev Neurobiol. 2010. PMID: 21095465 Review.
-
General properties and clinical possibilities of new selective inhibitors of catechol O-methyltransferase.Gen Pharmacol. 1994 Sep;25(5):813-24. doi: 10.1016/0306-3623(94)90082-5. Gen Pharmacol. 1994. PMID: 7835624 Review.
Cited by
-
Injury-Dependent and Disability-Specific Lumbar Spinal Gene Regulation following Sciatic Nerve Injury in the Rat.PLoS One. 2015 Apr 23;10(4):e0124755. doi: 10.1371/journal.pone.0124755. eCollection 2015. PLoS One. 2015. PMID: 25905723 Free PMC article.
-
Common variants of catechol-O-methyltransferase influence patient-controlled analgesia usage and postoperative pain in patients undergoing total hysterectomy.Pharmacogenomics J. 2016 Apr;16(2):186-92. doi: 10.1038/tpj.2015.33. Epub 2015 May 12. Pharmacogenomics J. 2016. PMID: 25963335
-
Interspecies comparison in the COMT-mediated methylation of 3-BTD.RSC Adv. 2018 May 1;8(29):16278-16284. doi: 10.1039/c8ra01938j. eCollection 2018 Apr 27. RSC Adv. 2018. PMID: 35542223 Free PMC article.
-
Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation.Brain Behav Immun. 2015 Nov;50:196-202. doi: 10.1016/j.bbi.2015.07.014. Epub 2015 Jul 15. Brain Behav Immun. 2015. PMID: 26187567 Free PMC article.
-
Sustained stimulation of β2- and β3-adrenergic receptors leads to persistent functional pain and neuroinflammation.Brain Behav Immun. 2018 Oct;73:520-532. doi: 10.1016/j.bbi.2018.06.017. Epub 2018 Jun 20. Brain Behav Immun. 2018. PMID: 29935309 Free PMC article.
References
-
- Bäckström R, Honkanen E, Pippuri A, Kairisalo P, Pystynen J, Heinola K, et al. Synthesis of some novel potent and selective catechol O-methyltransferase inhibitors. J Med Chem. 1989;32:841–846. - PubMed
-
- Beiske AG, Loge JH, Ronningen A, Svensson E. Pain in Parkinson's disease: prevalence and characteristics. Pain. 2009;141:173–177. - PubMed
-
- Berthele A, Platzer S, Jochim B, Boecker H, Buettner A, Conrad B, et al. COMT Val108/158Met genotype affects the mu-opioid receptor system in the human brain: evidence from ligand-binding, G-protein activation and preproenkephalin mRNA expression. Neuroimage. 2005;28:185–193. - PubMed
-
- Boudikova B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clin Pharmacol Ther. 1990;48:381–389. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

