Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation
- PMID: 20727173
- PMCID: PMC2939565
- DOI: 10.1186/1471-213X-10-89
Pax2 and Pax8 cooperate in mouse inner ear morphogenesis and innervation
Abstract
Background: Pax2;5;8 transcription factors play diverse roles in vertebrate and invertebrate organogenesis, including the development of the inner ear. Past research has suggested various cochlear defects and some vestibular defects in Pax2 null mice but the details of the cochlear defects and the interaction with other Pax family members in ear development remain unclear.
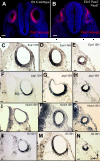
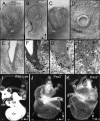
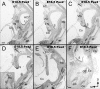
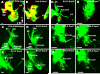
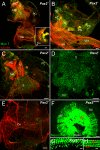
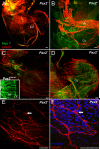
Results: We show that Pax2;8 double null mice do not develop an ear past the otocyst stage and show little to no sensory as well as limited and transient neuronal development, thus indicating that these two family members are essential for overall ear morphogenesis and sustained neurosensory development. In support of functional redundancy between Pax proteins, Pax2 can be substituted by a Pax5 minigene, a gene normally not expressed in the embryonic mouse ear. There is no detectable morphological defect in Pax8 null mice suggesting that Pax2 expression can compensate for Pax8. Conversely, Pax8 cannot compensate for Pax2 leading to a cochlear phenotype not fully appreciated previously: Cochlear development is delayed until E15.5 when the cochlea extrudes as a large sack into the brain case. Immunocytochemistry and tracing from the brain show that a cochlear spiral ganglia form as a small addition to the inferior vestibular ganglion. However, the empty cochlear sack, devoid of any sensory epithelium development as indicated by the absence of Sox2 or MyoVII expression, nevertheless develop a dense innervation network of small neurons situated in the wall of the cochlear sack.
Conclusions: Combined these data suggest that Pax2 is needed for organ of Corti formation and is directly or indirectly involved in the coordination of spiral ganglion formation which is partially disrupted in the Pax2 null ears. All three Pax genes can signal redundantly in the ear with their function being determined primarily by the spatio-temporal expression driven by the three distinct promoters of these genes.
Figures


References
-
- Favor J, Sandulache R, Neuhauser-Klaus A, Pretsch W, Chatterjee B, Senft E, Wurst W, Blanquet V, Grimes P, Sporle R. et al.The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc Natl Acad Sci USA. 1996;93(24):13870–13875. doi: 10.1073/pnas.93.24.13870. - DOI - PMC - PubMed
-
- Torres M, Gomez-Pardo E, Gruss P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development. 1996;122(11):3381–3391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

