Nicotine-induced survival signaling in lung cancer cells is dependent on their p53 status while its down-regulation by curcumin is independent
- PMID: 20727180
- PMCID: PMC2936340
- DOI: 10.1186/1476-4598-9-220
Nicotine-induced survival signaling in lung cancer cells is dependent on their p53 status while its down-regulation by curcumin is independent
Abstract
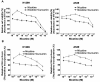
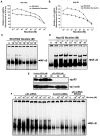
Background: Lung cancer is the most lethal cancer and almost 90% of lung cancer is due to cigarette smoking. Even though nicotine, one of the major ingredients of cigarette smoke and the causative agent for addiction, is not a carcinogen by itself, several investigators have shown that nicotine can induce cell proliferation and angiogenesis. We observed that the proliferative index of nicotine is different in the lung cancer cell lines H1299 (p53-/-) and A549 (p53+/+) which indicates that the mode of up-regulation of survival signals by nicotine might be different in cells with and without p53.
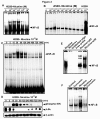
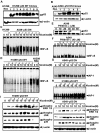
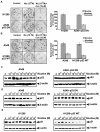
Results: While low concentrations of nicotine induced activation of NF-κB, Akt, Bcl2, MAPKs, AP1 and IAPs in H1299, it failed to induce NF-κB in A549, and compared to H1299, almost 100 times higher concentration of nicotine was required to induce all other survival signals in A549. Transfection of WT-p53 and DN-p53 in H1299 and A549 respectively, reversed the mode of activation of survival signals. Curcumin down-regulated all the survival signals induced by nicotine in both the cells, irrespective of their p53 status. The hypothesis was confirmed when lower concentrations of nicotine induced NF-κB in two more lung cancer cells, Hop-92 and NCI-H522 with mutant p53 status. Silencing of p53 in A549 using siRNA made the cells susceptible to nicotine-induced NF-κB nuclear translocation as in A549 DN-p53 cells.
Conclusions: The present study reveals a detrimental role of nicotine especially in lung cancer patients with impaired p53 status and identifies curcumin as a potential chemopreventive.
Figures



References
-
- D'Amico D, Carbone D, Mitsudomi T, Nau M, Fedorko J, Russell E, Johnson B, Buchhagen D, Bodner S, Phelps R, Gazdar A, Minna JD. High frequency of somatically acquired p53 mutations in small-cell lung cancer cell lines and tumors. Oncogene. 1992;7:339–346. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

