Nanomaterial cytotoxicity is composition, size, and cell type dependent
- PMID: 20727197
- PMCID: PMC2936333
- DOI: 10.1186/1743-8977-7-22
Nanomaterial cytotoxicity is composition, size, and cell type dependent
Abstract
Background: Despite intensive research efforts, reports of cellular responses to nanomaterials are often inconsistent and even contradictory. Additionally, relationships between the responding cell type and nanomaterial properties are not well understood. Using three model cell lines representing different physiological compartments and nanomaterials of different compositions and sizes, we have systematically investigated the influence of nanomaterial properties on the degrees and pathways of cytotoxicity. In this study, we selected nanomaterials of different compositions (TiO2 and SiO2 nanoparticles, and multi-wall carbon nanotubes [MWCNTs]) with differing size (MWCNTs of different diameters < 8 nm, 20-30 nm, > 50 nm; but same length 0.5-2 microm) to analyze the effects of composition and size on toxicity to 3T3 fibroblasts, RAW 264.7 macrophages, and telomerase-immortalized (hT) bronchiolar epithelial cells.
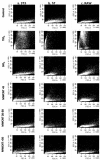
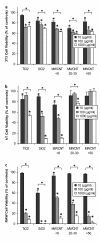
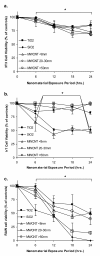
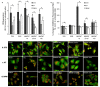
Results: Following characterization of nanomaterial properties in PBS and serum containing solutions, cells were exposed to nanomaterials of differing compositions and sizes, with cytotoxicity monitored through reduction in mitochondrial activity. In addition to cytotoxicity, the cellular response to nanomaterials was characterized by quantifying generation of reactive oxygen species, lysosomal membrane destabilization and mitochondrial permeability. The effect of these responses on cellular fate - apoptosis or necrosis - was then analyzed. Nanomaterial toxicity was variable based on exposed cell type and dependent on nanomaterial composition and size. In addition, nanomaterial exposure led to cell type dependent intracellular responses resulting in unique breakdown of cellular functions for each nanomaterial: cell combination.
Conclusions: Nanomaterials induce cell specific responses resulting in variable toxicity and subsequent cell fate based on the type of exposed cell. Our results indicate that the composition and size of nanomaterials as well as the target cell type are critical determinants of intracellular responses, degree of cytotoxicity and potential mechanisms of toxicity.
Figures
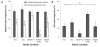
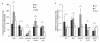
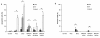
Similar articles
-
Cytotoxicity screening of 23 engineered nanomaterials using a test matrix of ten cell lines and three different assays.Part Fibre Toxicol. 2011 Feb 23;8:9. doi: 10.1186/1743-8977-8-9. Part Fibre Toxicol. 2011. PMID: 21345205 Free PMC article.
-
Systematic in vitro nanotoxicity study on anodic alumina nanotubes with engineered aspect ratio: understanding nanotoxicity by a nanomaterial model.Biomaterials. 2015 Apr;46:117-30. doi: 10.1016/j.biomaterials.2014.12.008. Epub 2015 Jan 23. Biomaterials. 2015. PMID: 25678121
-
Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials.Part Fibre Toxicol. 2019 Apr 11;16(1):18. doi: 10.1186/s12989-019-0299-z. Part Fibre Toxicol. 2019. PMID: 30975174 Free PMC article. Review.
-
Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species.J Phys Chem B. 2008 Oct 30;112(43):13608-19. doi: 10.1021/jp712087m. Epub 2008 Oct 3. J Phys Chem B. 2008. PMID: 18831567
-
Pro-Death or Pro-Survival: Contrasting Paradigms on Nanomaterial-Induced Autophagy and Exploitations for Cancer Therapy.Acc Chem Res. 2019 Nov 19;52(11):3164-3176. doi: 10.1021/acs.accounts.9b00397. Epub 2019 Oct 17. Acc Chem Res. 2019. PMID: 31621285 Review.
Cited by
-
IC50 Evaluation of Platinum Nanocatalysts for Cancer Treatment in Fibroblast, HeLa, and DU-145 Cell Lines.ACS Omega. 2020 Sep 23;5(39):25381-25389. doi: 10.1021/acsomega.0c03759. eCollection 2020 Oct 6. ACS Omega. 2020. PMID: 33043218 Free PMC article.
-
Cellular interaction and toxicity depend on physicochemical properties and surface modification of redox-active nanomaterials.ACS Nano. 2013 Jun 25;7(6):4855-68. doi: 10.1021/nn305872d. Epub 2013 May 23. ACS Nano. 2013. PMID: 23668322 Free PMC article.
-
Cytotoxicity investigation of luminescent nanohybrids based on chitosan and carboxymethyl chitosan conjugated with Bi2S3 quantum dots for biomedical applications.Toxicol Res (Camb). 2016 Apr 14;5(4):1017-1028. doi: 10.1039/c6tx00039h. eCollection 2016 Jul 1. Toxicol Res (Camb). 2016. PMID: 30090409 Free PMC article.
-
Single wall and multiwall carbon nanotubes induce different toxicological responses in rat alveolar macrophages.J Appl Toxicol. 2019 May;39(5):764-772. doi: 10.1002/jat.3765. Epub 2019 Jan 3. J Appl Toxicol. 2019. PMID: 30605223 Free PMC article.
-
Evaluation of the elastic Young's modulus and cytotoxicity variations in fibroblasts exposed to carbon-based nanomaterials.J Nanobiotechnology. 2019 Feb 23;17(1):32. doi: 10.1186/s12951-019-0460-8. J Nanobiotechnology. 2019. PMID: 30797235 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

