Fucoidan present in brown algae induces apoptosis of human colon cancer cells
- PMID: 20727207
- PMCID: PMC2931458
- DOI: 10.1186/1471-230X-10-96
Fucoidan present in brown algae induces apoptosis of human colon cancer cells
Abstract
Background: Fucoidan is a sulfated polysaccharide found in brown algae; it has been shown to exhibit a number of biological effects, including anti-tumor effects. In this study, we evaluated the effects of fucoidan on apoptosis in HT-29 and HCT116 human colon cancer cells.
Methods: HT-29 and HCT116 cells were cultured with various concentrations of fucoidan (0 - 20 microg/mL). Apoptosis was assayed via Hoechst staining and Annexin V staining followed by flow cytometric analysis. Western blot analyses and JC-1 staining were conducted to determine the levels of apoptosis-regulating proteins and mitochondrial membrane permeability, respectively.
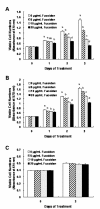
Results: Fucoidan induced substantial reductions in viable cell numbers and apoptosis of HT-29 and HCT116 cells in a dose-dependent manner. In HT-29 cells, fucoidan also increased the levels of cleaved caspases-8, -9, -7, and -3, and cleaved poly (ADP-ribose) polymerase (PARP) levels. The levels of the X-linked inhibitor of apoptosis protein and survivin were attenuated in the fucoidan-treated cells. Fucoidan was also shown to enhance mitochondrial membrane permeability, as well as the cytochrome c and Smac/Diablo release from the mitochondria. Fucoidan increased the levels of the Bak and truncated Bid proteins, but reduced the levels of Mcl-1. Additionally, fucoidan increased the levels of the tumor necrosis factor-related apoptosis-inducing ligand, Fas and death receptor 5 proteins. The caspase-8 and -9 inhibitors Z-IETD-FMK and Z-LEHD-FMK induced a reduction in fucoidan-mediated apoptosis. Caspase-8 inhibitor inhibited the fucoidan-induced cleavage of Bid, caspases-9 and -3, and PARP.
Conclusion: The findings of this study indicate that fucoidan induces apoptosis in HT-29 and HCT116 human colon cancer cells, and that this phenomenon is mediated via both the death receptor-mediated and mitochondria-mediated apoptotic pathways. These results suggest that fucoidan may prove useful in the development of a colon cancer-preventive protocol.
Figures





Similar articles
-
Induction of p53 contributes to apoptosis of HCT-116 human colon cancer cells induced by the dietary compound fisetin.Am J Physiol Gastrointest Liver Physiol. 2009 May;296(5):G1060-8. doi: 10.1152/ajpgi.90490.2008. Epub 2009 Mar 5. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19264955
-
Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells.Biol Pharm Bull. 2009 Oct;32(10):1760-4. doi: 10.1248/bpb.32.1760. Biol Pharm Bull. 2009. PMID: 19801840
-
Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human colon cancer cells.Clin Cancer Res. 2004 Dec 15;10(24):8284-92. doi: 10.1158/1078-0432.CCR-04-1289. Clin Cancer Res. 2004. PMID: 15623604
-
The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran.Carbohydr Polym. 2017 Dec 1;177:451-459. doi: 10.1016/j.carbpol.2017.09.005. Epub 2017 Sep 5. Carbohydr Polym. 2017. PMID: 28962791 Review.
-
The Therapeutic Potential of the Anticancer Activity of Fucoidan: Current Advances and Hurdles.Mar Drugs. 2021 May 10;19(5):265. doi: 10.3390/md19050265. Mar Drugs. 2021. PMID: 34068561 Free PMC article. Review.
Cited by
-
Screening of Dengue virus antiviral activity of marine seaweeds by an in situ enzyme-linked immunosorbent assay.PLoS One. 2012;7(12):e51089. doi: 10.1371/journal.pone.0051089. Epub 2012 Dec 5. PLoS One. 2012. PMID: 23227238 Free PMC article.
-
Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo.PLoS One. 2012;7(8):e43483. doi: 10.1371/journal.pone.0043483. Epub 2012 Aug 20. PLoS One. 2012. PMID: 22916270 Free PMC article.
-
Anticancer Effect of Sargassum oligocystom Hydroalcoholic Extract Against SW742, HT-29, WiDr, and CT-26 Colorectal Cancer Cell Lines and Expression of P53 and APC Genes.J Gastrointest Cancer. 2023 Mar;54(1):62-66. doi: 10.1007/s12029-021-00765-0. Epub 2022 Jan 9. J Gastrointest Cancer. 2023. PMID: 35000070
-
Chemical Composition, Antioxidant, and Antitumor Activity of Fucoidan from the Brown Alga Dictyota dichotoma.Molecules. 2023 Oct 19;28(20):7175. doi: 10.3390/molecules28207175. Molecules. 2023. PMID: 37894655 Free PMC article.
-
Metabolic and enzymatic elucidation of cooperative degradation of red seaweed agarose by two human gut bacteria.Sci Rep. 2021 Jul 6;11(1):13955. doi: 10.1038/s41598-021-92872-y. Sci Rep. 2021. PMID: 34230500 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

