The neuroendocrine basis of lactation-induced suppression of GnRH: role of kisspeptin and leptin
- PMID: 20727862
- PMCID: PMC3118984
- DOI: 10.1016/j.brainres.2010.08.038
The neuroendocrine basis of lactation-induced suppression of GnRH: role of kisspeptin and leptin
Abstract
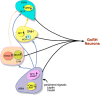
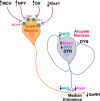
Lactation is an important physiological model of the integration of energy balance and reproduction, as it involves activation of potent appetitive neuropeptide systems coupled to a profound inhibition of pulsatile GnRH/LH secretion. There are multiple systems that contribute to the chronic hyperphagia of lactation: 1) suppression of the metabolic hormones, leptin and insulin, 2) activation of hypothalamic orexigenic neuropeptide systems NPY, AGRP, orexin (OX) and melanin concentrating hormone (MCH), 3) special induction of NPY expression in the dorsomedial hypothalamus, and 4) suppression of anorexigenic systems POMC and CART. These changes ensure adequate energy intake to meet the metabolic needs of milk production. There is significant overlap in all of the systems that regulate food intake with the regulation of GnRH, suggesting there could be several redundant factors acting to suppress GnRH/LH during lactation. In addition to an overall increase in inhibitory tone acting directly on GnRH cell bodies that is brought about by increases in orexigenic systems, there are also effects at the ARH to disrupt Kiss1/neurokinin B/dynorphin neuronal function through inhibition of Kiss1 and NKB. These changes could lead to an increase in inhibitory auto-regulation of the Kiss1 neurons and a possible disruption of pulsatile GnRH release. While the low levels of leptin and insulin contribute to the changes in ARH appetitive systems, they do not appear to contribute to the suppression of ARH Kiss1 or NKB. The inhibition of Kiss1 may be the key factor in the suppression of GnRH during lactation, although the mechanisms responsible for its inhibition are unknown.
Copyright © 2010. Published by Elsevier B.V.
Figures
References
-
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996:382. - PubMed
-
- Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology. 2007;132:2103–2115. - PubMed
-
- Barash IA, Cheung CC, Weigle DS, Ren HP, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. - PubMed
-
- Bellinger LL, Bernardis LL. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol. Behav. 2002;76:431–442. - PubMed
-
- Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin as adiposity signals. Recent Prog. Horm. Res. 2004;59:267–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

