MT1-MMP-mediated basement membrane remodeling modulates renal development
- PMID: 20727881
- PMCID: PMC2945451
- DOI: 10.1016/j.yexcr.2010.08.003
MT1-MMP-mediated basement membrane remodeling modulates renal development
Abstract
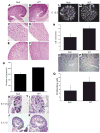
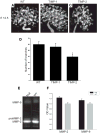
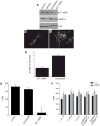
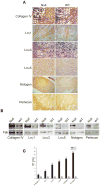
Extracellular matrix (ECM) remodeling regulates multiple cellular functions required for normal development and tissue repair. Matrix metalloproteinases (MMPs) are key mediators of this process and membrane targeted MMPs (MT-MMPs) in particular have been shown to be important in normal development of specific organs. In this study we investigated the role of MT1-MMP in kidney development. We demonstrate that loss of MT1-MMP leads to a renal phenotype characterized by a moderate decrease in ureteric bud branching morphogenesis and a severe proliferation defect. The kidneys of MT1-MMP-null mice have increased deposition of collagen IV, laminins, perlecan, and nidogen and the phenotype is independent of the MT-1MMP target, MMP-2. Utilizing in vitro systems we demonstrated that MTI-MMP proteolytic activity is required for renal tubule cells to proliferate in three dimensional matrices and to migrate on collagen IV and laminins. Together these data suggest an important role for MT1-MMP in kidney development, which is mediated by its ability to regulate cell proliferation and migration by proteolytically cleaving kidney basement membrane components.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
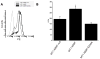
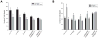
Similar articles
-
Tumor cell invasion of collagen matrices requires coordinate lipid agonist-induced G-protein and membrane-type matrix metalloproteinase-1-dependent signaling.Mol Cancer. 2006 Dec 8;5:69. doi: 10.1186/1476-4598-5-69. Mol Cancer. 2006. PMID: 17156449 Free PMC article.
-
Matrix metalloproteinases and their inhibitors regulate in vitro ureteric bud branching morphogenesis.Am J Physiol Renal Physiol. 2000 Nov;279(5):F891-900. doi: 10.1152/ajprenal.2000.279.5.F891. Am J Physiol Renal Physiol. 2000. PMID: 11053050
-
Stroma-derived matrix metalloproteinase (MMP)-2 promotes membrane type 1-MMP-dependent tumor growth in mice.Cancer Res. 2007 May 1;67(9):4311-9. doi: 10.1158/0008-5472.CAN-06-4761. Cancer Res. 2007. PMID: 17483344
-
The membrane tethered matrix metalloproteinase MT1-MMP at the forefront of melanoma cell invasion and metastasis.Pharmacol Res. 2016 Sep;111:17-22. doi: 10.1016/j.phrs.2016.05.019. Epub 2016 May 21. Pharmacol Res. 2016. PMID: 27221755 Review.
-
Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia.J Cell Sci. 2009 Sep 1;122(Pt 17):3015-24. doi: 10.1242/jcs.034561. J Cell Sci. 2009. PMID: 19692588 Review.
Cited by
-
Membrane-type 4 matrix metalloproteinase (MT4-MMP) modulates water homeostasis in mice.PLoS One. 2011 Feb 11;6(2):e17099. doi: 10.1371/journal.pone.0017099. PLoS One. 2011. PMID: 21347258 Free PMC article.
-
Transmembrane/cytoplasmic, rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary branching morphogenesis via binding to integrin β1.Development. 2013 Jan 15;140(2):343-52. doi: 10.1242/dev.084236. Development. 2013. PMID: 23250208 Free PMC article.
-
CLIC4 regulates late endosomal trafficking and matrix degradation activity of MMP14 at focal adhesions in RPE cells.Sci Rep. 2019 Aug 22;9(1):12247. doi: 10.1038/s41598-019-48438-0. Sci Rep. 2019. PMID: 31439888 Free PMC article.
-
Extracellular matrix determinants and the regulation of cancer cell invasion stratagems.J Microsc. 2013 Sep;251(3):250-60. doi: 10.1111/jmi.12064. J Microsc. 2013. PMID: 23924043 Free PMC article. Review.
-
Beyond tissue injury-damage-associated molecular patterns, toll-like receptors, and inflammasomes also drive regeneration and fibrosis.J Am Soc Nephrol. 2014 Jul;25(7):1387-400. doi: 10.1681/ASN.2014010117. Epub 2014 Apr 24. J Am Soc Nephrol. 2014. PMID: 24762401 Free PMC article. Review.
References
-
- Miner J. Renal basement membrane components. Kidney Int. 1999;56:2016–2024. - PubMed
-
- Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. - PubMed
-
- Kanwar OKYS, Yang Q, Wada J, Kashihara N, Tian Y, Wallner EI. Role of membrane-type matrix metalloproteinase 1 (MT-1-MMP), MMP-2, and its inhibitor in nephrogenesis. Am J Physiol. 1999;277:934–947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

