The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds
- PMID: 20728934
- PMCID: PMC2956268
- DOI: 10.1016/j.biomaterials.2010.07.083
The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds
Abstract
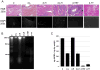
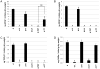
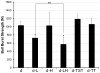
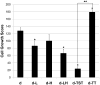
Biologic materials from various species and tissues are commonly used as surgical meshes or scaffolds for tissue reconstruction. Extracellular matrix (ECM) represents the secreted product of the cells comprising each tissue and organ, and therefore provides a unique biologic material for selected regenerative medicine applications. Minimal disruption of ECM ultrastructure and content during tissue processing is typically desirable. The objective of this study was to systematically evaluate effects of commonly used tissue processing steps upon porcine dermal ECM scaffold composition, mechanical properties, and cytocompatibility. Processing steps evaluated included liming and hot water sanitation, trypsin/SDS/TritonX-100 decellularization, and trypsin/TritonX-100 decellularization. Liming decreased the growth factor and glycosaminoglycan content, the mechanical strength, and the ability of the ECM to support in vitro cell growth (p ≤ 0.05 for all). Hot water sanitation treatment decreased only the growth factor content of the ECM (p ≤ 0.05). Trypsin/SDS/TritonX-100 decellularization decreased the growth factor content and the ability of the ECM to support in vitro cell growth (p ≤ 0.05 for both). Trypsin/Triton X-100 decellularization also decreased the growth factor content of the ECM but increased the ability of the ECM to support in vitro cell growth (p ≤ 0.05 for both). We conclude that processing steps evaluated in the present study affect content, mechanical strength, and/or cytocompatibility of the resultant porcine dermal ECM, and therefore care must be taken in choosing appropriate processing steps to maintain the beneficial effects of ECM in biologic scaffolds.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Decellularization of fibroblast cell sheets for natural extracellular matrix scaffold preparation.Tissue Eng Part C Methods. 2015 Jan;21(1):77-87. doi: 10.1089/ten.tec.2013.0666. Tissue Eng Part C Methods. 2015. PMID: 24866751 Free PMC article.
-
Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix.Tissue Eng Part C Methods. 2011 Apr;17(4):411-21. doi: 10.1089/ten.TEC.2010.0342. Epub 2011 Feb 5. Tissue Eng Part C Methods. 2011. PMID: 21043998 Free PMC article.
-
Enhanced decellularization technique of porcine dermal ECM for tissue engineering applications.Mater Sci Eng C Mater Biol Appl. 2019 Nov;104:109841. doi: 10.1016/j.msec.2019.109841. Epub 2019 Jun 6. Mater Sci Eng C Mater Biol Appl. 2019. PMID: 31499993
-
[Recent research progress of decellularization of native tissues].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2012 Oct;29(5):1007-13. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2012. PMID: 23198451 Review. Chinese.
-
Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance.Methods. 2015 Aug;84:25-34. doi: 10.1016/j.ymeth.2015.03.005. Epub 2015 Mar 16. Methods. 2015. PMID: 25791470 Review.
Cited by
-
Functional Scaffolds for Bone Tissue Regeneration: A Comprehensive Review of Materials, Methods, and Future Directions.J Funct Biomater. 2024 Sep 25;15(10):280. doi: 10.3390/jfb15100280. J Funct Biomater. 2024. PMID: 39452579 Free PMC article. Review.
-
Comparative biology of decellularized lung matrix: Implications of species mismatch in regenerative medicine.Biomaterials. 2016 Sep;102:220-30. doi: 10.1016/j.biomaterials.2016.06.025. Epub 2016 Jun 16. Biomaterials. 2016. PMID: 27344365 Free PMC article.
-
Biological evaluations of decellularized extracellular matrix collagen microparticles prepared based on plant enzymes and aqueous two-phase method.Regen Biomater. 2021 Mar 13;8(2):rbab002. doi: 10.1093/rb/rbab002. eCollection 2021 Mar. Regen Biomater. 2021. PMID: 33738116 Free PMC article.
-
Interdisciplinary Methods for Zoonotic Tissue Acellularization for Natural Heart Valve Substitute of Biomimetic Materials.Materials (Basel). 2022 Apr 1;15(7):2594. doi: 10.3390/ma15072594. Materials (Basel). 2022. PMID: 35407927 Free PMC article.
-
[Effect of pH on the chelation between strontium ions and decellularized small intestinal submucosal sponge scaffolds].Beijing Da Xue Xue Bao Yi Xue Ban. 2023 Feb 18;55(1):44-51. doi: 10.19723/j.issn.1671-167X.2023.01.007. Beijing Da Xue Xue Bao Yi Xue Ban. 2023. PMID: 36718688 Free PMC article. Chinese.
References
-
- Armour AD, Fish JS, Woodhouse KA, Semple JL. A comparison of human and porcine acellularized dermis: interactions with human fibroblasts in vitro. Plast Reconstr Surg. 2006;117(3):845–856. - PubMed
-
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–238. - PubMed
-
- Walles T, Herden T, Haverich A, Mertsching H. Influence of scaffold thickness and scaffold composition on bioartificial graft survival. Biomaterials. 2003;24(7):1233–1239. - PubMed
-
- Cebotari S, Lichtenberg A, Tudorache I, Hilfiker A, Mertsching H, Leyh R, et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation. 2006;114(1 Suppl):I132–137. - PubMed
-
- Lichtenberg A, Tudorache I, Cebotari S, Suprunov M, Tudorache G, Goerler H, et al. Preclinical testing of tissue-engineered heart valves re-endothelialized under simulated physiological conditions. Circulation. 2006;114(1 Suppl):I559–565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

