A phase I factorial design study of dose-dense temozolomide alone and in combination with thalidomide, isotretinoin, and/or celecoxib as postchemoradiation adjuvant therapy for newly diagnosed glioblastoma
- PMID: 20729242
- PMCID: PMC3098026
- DOI: 10.1093/neuonc/noq100
A phase I factorial design study of dose-dense temozolomide alone and in combination with thalidomide, isotretinoin, and/or celecoxib as postchemoradiation adjuvant therapy for newly diagnosed glioblastoma
Abstract
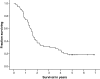
External beam radiation therapy (XRT) with concomitant temozolomide and 6 cycles of adjuvant temozolomide (5/28-day schedule) improves survival in patients with newly diagnosed glioblastoma compared with XRT alone. Studies suggest that dose-dense temozolomide schedules and addition of cytostatic agents may further improve efficacy. This factorial design phase I/II protocol tested dose-dense temozolomide alone and combined with cytostatic agents. Patients with newly diagnosed glioblastoma received fractionated XRT to 60 Gy concomitant with temozolomide (75 mg/m²)/day for 42 days). In the phase I portion, patients with stable disease or radiologic response 1 month after chemoradiation were randomized to adjuvant temozolomide alone (150 mg/m²/day, 7/14-day schedule) or with doublet combinations of thalidomide (400 mg/day), isotretinoin (100 mg/m²/day), and/or celecoxib (400 mg twice daily), or all 3 agents. Toxicity was assessed after 4 weeks. Among 54 patients enrolled (median age, 52 years; median Karnofsky performance status, 90), adjuvant treatment was not administered to 12 (22%), primarily because of disease progression (n = 10). All combinations were well tolerated. Grade 3/4 lymphopenia developed in 63% of patients, but no related infections occurred. One patient treated with temozolomide plus isotretinoin plus thalidomide had dose-limiting grade 3 fatigue and rash, and 1 patient receiving all 4 agents had dose-limiting grade 4 neutropenia. Venous thrombosis occurred in 7 patients, 4 of whom received thalidomide. From study entry, median survival was 20 months and the 2-year survival rate was 40%. Multiple cytostatic agents can be safely combined with dose-dense temozolomide. The factorial-based phase II portion of this study is currently ongoing.
Figures
Similar articles
-
Randomized phase II adjuvant factorial study of dose-dense temozolomide alone and in combination with isotretinoin, celecoxib, and/or thalidomide for glioblastoma.Neuro Oncol. 2015 Feb;17(2):266-73. doi: 10.1093/neuonc/nou155. Epub 2014 Sep 19. Neuro Oncol. 2015. PMID: 25239666 Free PMC article. Clinical Trial.
-
Phase II study of temozolomide, thalidomide, and celecoxib for newly diagnosed glioblastoma in adults.Neuro Oncol. 2008 Jun;10(3):300-8. doi: 10.1215/15228517-2008-005. Epub 2008 Apr 10. Neuro Oncol. 2008. PMID: 18403492 Free PMC article. Clinical Trial.
-
A phase II study of concurrent temozolomide and cis-retinoic acid with radiation for adult patients with newly diagnosed supratentorial glioblastoma.Int J Radiat Oncol Biol Phys. 2005 Apr 1;61(5):1454-9. doi: 10.1016/j.ijrobp.2004.08.023. Int J Radiat Oncol Biol Phys. 2005. PMID: 15817350 Clinical Trial.
-
A review of the economic burden of glioblastoma and the cost effectiveness of pharmacologic treatments.Pharmacoeconomics. 2014 Dec;32(12):1201-12. doi: 10.1007/s40273-014-0198-y. Pharmacoeconomics. 2014. PMID: 25085219 Review.
-
Adding high-dose celecoxib to increase effectiveness of standard glioblastoma chemoirradiation.Ann Pharm Fr. 2021 Sep;79(5):481-488. doi: 10.1016/j.pharma.2021.03.001. Epub 2021 Mar 6. Ann Pharm Fr. 2021. PMID: 33689795 Review.
Cited by
-
Factorial clinical trials: a new approach to phase II neuro-oncology studies.Neuro Oncol. 2015 Feb;17(2):174-6. doi: 10.1093/neuonc/nou314. Epub 2014 Dec 1. Neuro Oncol. 2015. PMID: 25452393 Free PMC article. No abstract available.
-
Emerging Therapies for Glioblastoma.Cancers (Basel). 2024 Apr 12;16(8):1485. doi: 10.3390/cancers16081485. Cancers (Basel). 2024. PMID: 38672566 Free PMC article. Review.
-
Induction of selective blood-tumor barrier permeability and macromolecular transport by a biostable kinin B1 receptor agonist in a glioma rat model.PLoS One. 2012;7(5):e37485. doi: 10.1371/journal.pone.0037485. Epub 2012 May 21. PLoS One. 2012. PMID: 22629405 Free PMC article.
-
Phase 1 lead-in to a phase 2 factorial study of temozolomide plus memantine, mefloquine, and metformin as postradiation adjuvant therapy for newly diagnosed glioblastoma.Cancer. 2019 Feb 1;125(3):424-433. doi: 10.1002/cncr.31811. Epub 2018 Oct 25. Cancer. 2019. PMID: 30359477 Free PMC article. Clinical Trial.
-
Cyclooxygenase-2 in glioblastoma multiforme.Drug Discov Today. 2017 Jan;22(1):148-156. doi: 10.1016/j.drudis.2016.09.017. Epub 2016 Sep 28. Drug Discov Today. 2017. PMID: 27693715 Free PMC article. Review.
References
-
- CBTRUS. Statistical report: Primary brain tumors in the United States, 1997–2001. Central Brain Tumor Registry of the United States. 2004 http://www.cbtrus.org/reports//2004-2005/2005report.pdf . Accessed March 16, 2010.
-
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. - PubMed
-
- Gilbert MR, Friedman HS, Kuttesch JF, et al. A phase II study of temozolomide in patients with newly diagnosed supratentorial malignant glioma before radiation therapy. Neuro Oncol. 2002;4:261–267. doi:10.1215/15228517-4-4-261. - DOI - PMC - PubMed
-
- Stupp R, Dietrich PY, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375–1382. doi:10.1200/JCO.20.5.1375. - DOI - PubMed
-
- Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi:10.1054/bjoc.2000.1316. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical