Genetics and regulation of the major enzymes of alanine synthesis in Escherichia coli
- PMID: 20729367
- PMCID: PMC2950514
- DOI: 10.1128/JB.00738-10
Genetics and regulation of the major enzymes of alanine synthesis in Escherichia coli
Abstract
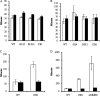
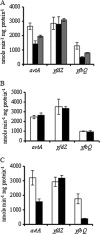
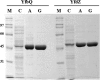
Genetic analysis of alanine synthesis in the model genetic organism Escherichia coli has implicated avtA, the still uncharacterized alaA and alaB genes, and probably other genes. We identified alaA as yfbQ. We then transferred mutations in several transaminase genes into a yfbQ mutant and isolated a mutant that required alanine for optimal growth. For cells grown with carbon sources other than pyruvate, the major alanine-synthesizing transaminases are AvtA, YfbQ (AlaA), and YfdZ (which we designate AlaC). Growth with pyruvate as the carbon source and multicopy suppression suggest that several other transaminases can contribute to alanine synthesis. Expression studies showed that alanine modestly repressed avtA and yfbQ but had no effect on yfdZ. The leucine-responsive regulatory protein (Lrp) mediated control by alanine. We purified YfbQ and YfdZ and showed that both are dimers with K(m)s for pyruvate within the intracellular range of pyruvate concentration.
Figures
References
-
- Berg, C. M., L. Liu, N. B. Vartak, W. A. Whalen, and B. Wang. 1990. The branched-chain amino acid transaminase genes and their products in Escherichia coli, p. 131-162. In Z. Barak, D. M. Chipman, and J. V. Scholl (ed.), Biosynthesis of branched chain amino acids. VCH Verlagsgesellschaft, Weinheim, Germany.
-
- Bondos, S. E., and A. Bicknell. 2003. Detection and prevention of protein aggregation before, during, and after purification. Anal. Biochem. 316:223-231. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

