Defining new insight into atypical arrhythmia: a computational model of ankyrin-B syndrome
- PMID: 20729400
- PMCID: PMC2993217
- DOI: 10.1152/ajpheart.00503.2010
Defining new insight into atypical arrhythmia: a computational model of ankyrin-B syndrome
Abstract
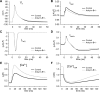
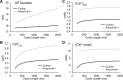
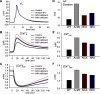
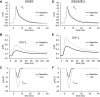
Normal cardiac excitability depends on the coordinated activity of specific ion channels and transporters within specialized domains at the plasma membrane and sarcoplasmic reticulum. Ion channel dysfunction due to congenital or acquired defects has been linked to human cardiac arrhythmia. More recently, defects in ion channel-associated proteins have been associated with arrhythmia. Ankyrin-B is a multifunctional adapter protein responsible for targeting select ion channels, transporters, cytoskeletal proteins, and signaling molecules in excitable cells, including neurons, pancreatic β-cells, and cardiomyocytes. Ankyrin-B dysfunction has been linked to cardiac arrhythmia in human patients and ankyrin-B heterozygous (ankyrin-B(+/-)) mice with a phenotype characterized by sinus node dysfunction, susceptibility to ventricular arrhythmias, and sudden death ("ankyrin-B syndrome"). At the cellular level, ankyrin-B(+/-) cells have defects in the expression and membrane localization of the Na(+)/Ca(2+) exchanger and Na(+)-K(+)-ATPase, Ca(2+) overload, and frequent afterdepolarizations, which likely serve as triggers for lethal cardiac arrhythmias. Despite knowledge gathered from mouse models and human patients, the molecular mechanism responsible for cardiac arrhythmias in the setting of ankyrin-B dysfunction remains unclear. Here, we use mathematical modeling to provide new insights into the cellular pathways responsible for Ca(2+) overload and afterdepolarizations in ankyrin-B(+/-) cells. We show that the Na(+)/Ca(2+) exchanger and Na(+)-K(+)-ATPase play related, yet distinct, roles in intracellular Ca(2+) accumulation, sarcoplasmic reticulum Ca(2+) overload, and afterdepolarization generation in ankyrin-B(+/-) cells. These findings provide important insights into the molecular mechanisms underlying a human disease and are relevant for acquired human arrhythmia, where ankyrin-B dysfunction has recently been identified.
Figures

References
-
- Ayalon G, Davis JQ, Scotland PB, Bennett V. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135: 1189– 1200, 2008. - PubMed
-
- Bhasin N, Cunha SR, Mduannayake M, Gigena MS, Rogers TB, Mohler PJ. Molecular basis for PP2A regulatory subunit B56α targeting in cardiomyocytes. Am J Physiol Heart Circ Physiol 293: H109– H119, 2007. - PubMed
-
- Bondarenko VE, Szigeti GP, Bett GC, Kim SJ, Rasmusson RL. Computer model of action potential of mouse ventricular myocytes. Am J Physiol Heart Circ Physiol 287: H1378– H1403, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

