Sphingosine-1-phosphate prevents permeability increases via activation of endothelial sphingosine-1-phosphate receptor 1 in rat venules
- PMID: 20729401
- PMCID: PMC2993214
- DOI: 10.1152/ajpheart.00462.2010
Sphingosine-1-phosphate prevents permeability increases via activation of endothelial sphingosine-1-phosphate receptor 1 in rat venules
Abstract
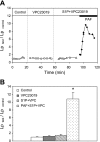
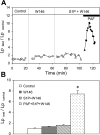
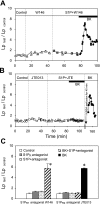
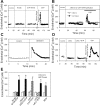
Sphingosine-1-phosphate (S1P) has been demonstrated to enhance endothelial barrier function in vivo and in vitro. However, different S1P receptor subtypes have been indicated to play different or even opposing roles in the regulation of vascular barrier function. This study aims to differentiate the roles of endogenous endothelial S1P subtype receptors in the regulation of permeability in intact microvessels using specific receptor agonist and antagonists. Microvessel permeability was measured with hydraulic conductivity (L(p)) in individually perfused rat mesenteric venules. S1P-mediated changes in endothelial intracellular Ca(2+) concentration ([Ca(2+)](i)) was measured in fura-2-loaded venules. Confocal images of fluorescent immunostaining illustrated the spatial expressions of three S1P subtype receptors (S1P(R1-3)) in rat venules. The application of S1P (1 μM) in the presence of S1P(R1-3) inhibited platelet-activating factor- or bradykinin-induced permeability increase. This S1P effect was reversed only with a selective S1P(R1) antagonist, W-146, and was not affected by S1P(R2) or S1P(R3) antagonists JTE-013 and CAY-10444, respectively. S1P(R1) was also identified as the sole receptor responsible for S1P-mediated increases in endothelial [Ca(2+)](i). S1P(R2) or S1P(R3) antagonist alone affected neither basal L(p) nor platelet-activating factor-induced permeability increase. The selective S1P(R1) agonist, SEW-2871, showed similar [Ca(2+)](i) and permeability effect to that of S1P. These results indicate that, despite the presence of S1P(R1-3) in the intact venules, only the activation of endothelial S1P(R1) is responsible for the protective action of S1P on microvessel permeability and that endogenous S1P(R2) or S1P(R3) did not exhibit functional roles in the regulation of permeability under basal or acutely stimulated conditions.
Figures





Similar articles
-
Sphingosine 1-phosphate prevents platelet-activating factor-induced increase in hydraulic conductivity in rat mesenteric venules: pertussis toxin sensitive.Am J Physiol Heart Circ Physiol. 2005 Aug;289(2):H840-4. doi: 10.1152/ajpheart.00026.2005. Epub 2005 Mar 18. Am J Physiol Heart Circ Physiol. 2005. PMID: 15778280
-
Erythrocyte-derived sphingosine-1-phosphate stabilizes basal hydraulic conductivity and solute permeability in rat microvessels.Am J Physiol Heart Circ Physiol. 2012 Oct 1;303(7):H825-34. doi: 10.1152/ajpheart.00181.2012. Epub 2012 Aug 3. Am J Physiol Heart Circ Physiol. 2012. PMID: 22865384 Free PMC article.
-
Effect of sphingosine 1-phosphate on morphological and functional responses in endothelia and venules after scalding injury.Burns. 2009 Dec;35(8):1171-9. doi: 10.1016/j.burns.2009.02.012. Epub 2009 Jun 10. Burns. 2009. PMID: 19520517
-
New players on the center stage: sphingosine 1-phosphate and its receptors as drug targets.Biochem Pharmacol. 2008 May 15;75(10):1893-900. doi: 10.1016/j.bcp.2007.12.018. Epub 2008 Jan 5. Biochem Pharmacol. 2008. PMID: 18321471 Review.
-
In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights.Cell Signal. 2005 Feb;17(2):131-9. doi: 10.1016/j.cellsig.2004.08.006. Cell Signal. 2005. PMID: 15494205 Review.
Cited by
-
Sphingosine-1-Phosphate Alleviates Irradiation Induced Salivary Gland Hypofunction through Preserving Endothelial Cells and Resident Macrophages.Antioxidants (Basel). 2022 Oct 18;11(10):2050. doi: 10.3390/antiox11102050. Antioxidants (Basel). 2022. PMID: 36290773 Free PMC article.
-
New insights into shear stress-induced endothelial signalling and barrier function: cell-free fluid versus blood flow.Cardiovasc Res. 2017 Apr 1;113(5):508-518. doi: 10.1093/cvr/cvx021. Cardiovasc Res. 2017. PMID: 28158679 Free PMC article.
-
Sphingosine 1-phosphate and its regulatory role in vascular endothelial cells.Histol Histopathol. 2022 Mar;37(3):213-225. doi: 10.14670/HH-18-428. Epub 2022 Feb 4. Histol Histopathol. 2022. PMID: 35118637 Review.
-
Sphingosine-1-phosphate induced contraction of bladder smooth muscle.Eur J Pharmacol. 2013 Nov 15;720(1-3):355-62. doi: 10.1016/j.ejphar.2013.10.004. Epub 2013 Oct 10. Eur J Pharmacol. 2013. PMID: 24120660 Free PMC article.
-
Activating Sphingosine-1-phospahte signaling in endothelial cells increases myosin light chain phosphorylation to decrease endothelial permeability thereby inhibiting cancer metastasis.Cancer Lett. 2021 May 28;506:107-119. doi: 10.1016/j.canlet.2021.01.004. Epub 2021 Feb 16. Cancer Lett. 2021. PMID: 33600895 Free PMC article.
References
-
- Adamson RH, Zeng M, Adamson GN, Lenz JF, Curry FE. PAF- and bradykinin-induced hyperpermeability of rat venules is independent of actin-myosin contraction. Am J Physiol Heart Circ Physiol 285: H406– H417, 2003 - PubMed
-
- Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516– F1524, 2006 - PubMed
-
- Curry PE, Huxley VH, Sarelius IH. Techniques in microcirculation: measurement of permeability, pressure and flow. In: Cardiovascular Physiology. Techniques in the Life Sciences. New York: Elsevier, 1983, p. 1–34
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

