New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy
- PMID: 20729419
- PMCID: PMC3130298
- DOI: 10.2214/AJR.09.3928
New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy
Abstract
Objective: The purpose of this article is to compare the recently published revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1) to the original guidelines (RECIST 1.0) for advanced non-small cell lung cancer (NSCLC) after erlotinib therapy and to evaluate the impact of the new CT tumor measurement guideline on response assessment.
Materials and methods: Forty-three chemotherapy-naive patients with advanced NSCLC treated with erlotinib in a single-arm phase 2 multicenter open-label clinical trial were retrospectively studied. CT tumor measurement records using RECIST 1.0 that were generated as part of the prospective clinical trial were reviewed. A second set of CT tumor measurements was generated from the records to meet RECIST 1.1 guidelines. The number of target lesions, best response, and time to progression were compared between RECIST 1.1 and RECIST 1.0.
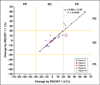
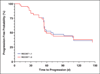
Results: The number of target lesions according to RECIST 1.1 decreased in 22 patients (51%) and did not change in 21 patients (49%) compared with the number according to RECIST 1.0 (p < 0.0001, paired Student's t test). Almost perfect agreement was observed between best responses using RECIST 1.1 and RECIST 1.0 (weighted kappa = 0.905). Two patients with stable disease according to RECIST 1.0 had progressive disease according to RECIST 1.1 criteria because of new lesions found on PET/CT. There was no significant difference in time to progression between RECIST 1.1 and RECIST 1.0 (p = 1.000, sign test).
Conclusion: RECIST 1.1 provided almost perfect agreement in response assessment after erlotinib therapy compared with RECIST 1.0. Assessment with PET/CT was a major factor that influenced the difference in best response assessment between RECIST 1.1 and RECIST 1.0.
Figures




References
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors (RECIST guidelines) J Natl Cancer Inst. 2000;92:205–216. - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumors: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. - PubMed
-
- Suzuki C, Jacobsson H, Hatschek T, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. RadioGraphics. 2008;28:329–344. - PubMed
-
- Bogaerts J, Ford R, Sargent D, et al. Individual patient data analysis to assess modifications to the RECIST criteria. Eur J Cancer. 2009;45:248–260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

