Pharmacology of store-operated calcium channels
- PMID: 20729487
- PMCID: PMC2965610
- DOI: 10.1124/mi.10.4.4
Pharmacology of store-operated calcium channels
Abstract
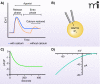
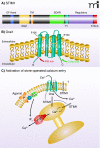
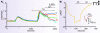
Store-operated calcium entry is a process by which the depletion of calcium from the endoplasmic reticulum activates calcium influx across the plasma membrane. In the past few years, the major players in this pathway have been identified. STIM1 and STIM2 function as calcium sensors in the endoplasmic reticulum and can interact with and activate plasma membrane channels comprised of Orai1, Orai2, or Orai3 subunits. This review discusses recent advances in our understanding of this widespread signaling mechanism as well as the mechanisms by which a number of interesting pharmacological agents modify it.
Figures

References
-
- Parekh AB, Putney JW. (2005) Store-operated calcium channels. Physiol Rev 85:57–810 - PubMed
-
- Putney JW. (1986) A model for receptor-regulated calcium entry. Cell Calcium 7:1–12 This hypothesis paper first described a model whereby depletion of endoplasmic calcium stores activated calcium permeable channels in the plasma membrane. - PubMed
-
- Putney JW. (2001) Pharmacology of capacitative calcium entry. Mol Interv 1:84–94 - PubMed
-
- Tsien RY. (1989) Fluorescent probes of cell signalling. Ann Rev Neurosci 12:227–253 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
