Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels
- PMID: 20729489
- PMCID: PMC2965611
- DOI: 10.1124/mi.10.4.6
Molecular mechanisms of SERT in platelets: regulation of plasma serotonin levels
Abstract
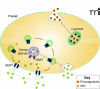
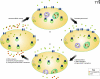
The serotonin transporter (SERT) on platelets is a primary mechanism for serotonin (5HT) uptake from the blood plasma. Alteration in plasma 5HT level is associated with a number of cardiovascular diseases and disorders. Therefore, the regulation of the transporter's activity represents a key mechanism to stabilize the concentration of plasma 5HT. There is a biphasic relationship between plasma 5HT elevation, loss of surface SERT, and depletion of platelet 5HT. Specifically, in platelets, plasma membrane SERT levels and platelet 5HT uptake initially rise as plasma 5HT levels are increased but then fall below normal as the plasma 5HT level continues to rise. Therefore, we propose that elevated plasma 5HT limits its own uptake in platelets by down-regulating SERT as well as modifying the characteristics of SERT partners in the membrane trafficking pathway. This review will summarize current findings regarding the biochemical mechanisms by which elevated 5HT downregulates the expression of SERT on the platelet membrane. Intriguing aspects of this regulation include the intracellular interplay of SERT with the small G protein Rab4 and the concerted 5HT-mediated phosphorylation of vimentin.
Figures
References
-
- Walther DJ, Bader M. (1999) Serotonin synthesis in murine embryonic stem cells. Mol Brain Res 68:55–63 The authors utilized embryonic stem cells to study early effects of serotonin during early embryonic ontogeny. - PubMed
-
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. (2003) Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37:233–247 Pet-1 is identified as a marker for 5-HT neurons and is expressed shortly before 5-HT appears in the hindbrain. - PubMed
-
- Walther DJ, Peter JU, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M. (2003) Synthesis of serotonin by a second tryptophan hydroxy-lase isoform. Science 299:76 This study provides the first evidence for the second tryptophan hydroxylase isoform. - PubMed
-
- Côté F, Thévenot E, Fligny C, et al. (2003) Disruption of the nonneuronal Tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A 100:13525–13530 TPH1 is established in this study to be responsible for the synthesis of peripheral tissue serotonin, demonstrating the importance of blood serotonin in cardiac function. - PMC - PubMed
-
- Vanhoutte PM. (1991) Platelet-derived serotonin, the endothelium, and cardiovascular disease. J Cardiovasc Pharmacol 17:S6–12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
