Antiretroviral release from poly(DL-lactide-co-glycolide) nanoparticles in mice
- PMID: 20729545
- PMCID: PMC2941676
- DOI: 10.1093/jac/dkq318
Antiretroviral release from poly(DL-lactide-co-glycolide) nanoparticles in mice
Abstract
Objectives: Free ritonavir, lopinavir and efavirenz injected intraperitoneally were compared with antiretroviral (AR) nanoparticles (NPs).
Methods: This is a prospective study in BALB/c mice comparing the pharmacokinetics of free drugs with AR NPs. All animals received free drugs or AR NPs (20 mg/kg) in PBS. In vitro replication assays were used for determination of the anti-HIV efficacy of NP formulations. At specific times (free drugs 0.08, 0.125, 0.25, 0.33, 1, 2 and 3 days; AR NPs 0.125, 0.33, 1, 2, 4, 7, 14, 21, 28, 35 and 42 days) mice were euthanized and serum and organs were harvested for determination of AR concentrations by HPLC. Single treatment of monocyte-derived macrophages (MDMs) infected with HIV-1(ada) compared AR NPs (0.005-0.05 mg/mL) with free efavirenz or lopinavir/ritonavir (0.01-0.1 mg/mL), blank NPs and controls. Results are presented as means ± SEM.
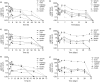
Results: Serum free AR drug concentrations peaked 4 h post-injection (ritonavir 3.9 ± 3.05, lopinavir 3.4 ± 2.5 and efavirenz 1.8 ± 0.63 µg/mL) and were eliminated by 72 h. Poly(dl-lactide-co-glycolide) NP animals had detectable ritonavir, lopinavir and efavirenz concentrations in all tissues for 28 days. Treatment of MDMs with AR NPs resulted in sustained inhibition of HIV-1(ada) replication.
Conclusions: AR drug concentrations from NPs are sustained for 28 days in vivo and anti-HIV inhibition was comparable to that of free drugs in vitro and could be a sustained treatment for delivery of AR drugs.
Figures

References
-
- Allavena C, Ferre V, Brunet-Francois C, et al. Efficacy and tolerability of a nucleoside reverse transcriptase inhibitor-sparing combination of lopinavir/ritonavir and efavirenz in HIV-1-infected patients. J Acquir Immune Defic Syndr. 2005;39:300–6. doi:10.1097/01.qai.0000165914.42827.bb. - DOI - PubMed
-
- Riddler SA, Haubrich R, DiRenzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. New Engl J Med. 2008;358:2095–106. doi:10.1056/NEJMoa074609. - DOI - PMC - PubMed
-
- Crowe S, Zhu T, Muller WE. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. J Leukoc Biol. 2003;74:635–41. doi:10.1189/jlb.0503204. - DOI - PubMed
-
- Anabwani G, Navario P. Nutrition and HIV/AIDS in sub-Saharan Africa: an overview. Nutrition. 2005;21:96–9. doi:10.1016/j.nut.2004.09.013. - DOI - PubMed
-
- Plot P, Bartos M, Ghys PD, et al. The global impact of HIV/AIDS. Nature. 2001;410:968–73. doi:10.1038/35073639. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

