Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing
- PMID: 20729546
- PMCID: PMC2963335
- DOI: 10.1074/jbc.M110.108175
Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing
Abstract
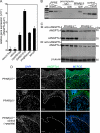
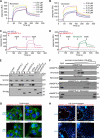
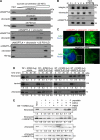
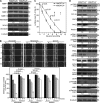
A dynamic cell-matrix interaction is crucial for a rapid cellular response to changes in the environment. Appropriate cell behavior in response to the changing wound environment is required for efficient wound closure. However, the way in which wound keratinocytes modify the wound environment to coordinate with such cellular responses remains less studied. We demonstrated that angiopoietin-like 4 (ANGPTL4) produced by wound keratinocytes coordinates cell-matrix communication. ANGPTL4 interacts with vitronectin and fibronectin in the wound bed, delaying their proteolytic degradation by metalloproteinases. This interaction does not interfere with integrin-matrix protein recognition and directly affects cell-matrix communication by altering the availability of intact matrix proteins. These interactions stimulate integrin- focal adhesion kinase, 14-3-3, and PKC-mediated signaling pathways essential for effective wound healing. The deficiency of ANGPTL4 in mice delays wound re-epithelialization. Further analysis revealed that cell migration was impaired in the ANGPTL4-deficient keratinocytes. Altogether, the findings provide molecular insight into a novel control of wound healing via ANGPTL4-dependent regulation of cell-matrix communication. Given the known role of ANGPTL4 in glucose and lipid homeostasis, it is a prime therapeutic candidate for the treatment of diabetic wounds. It also underscores the importance of cell-matrix communication during angiogenesis and cancer metastasis.
Figures
Similar articles
-
Angiopoietin-like 4 interacts with integrins beta1 and beta5 to modulate keratinocyte migration.Am J Pathol. 2010 Dec;177(6):2791-803. doi: 10.2353/ajpath.2010.100129. Epub 2010 Oct 15. Am J Pathol. 2010. PMID: 20952587 Free PMC article.
-
Angiopoietin-like 4 stimulates STAT3-mediated iNOS expression and enhances angiogenesis to accelerate wound healing in diabetic mice.Mol Ther. 2014 Sep;22(9):1593-604. doi: 10.1038/mt.2014.102. Epub 2014 Jun 6. Mol Ther. 2014. PMID: 24903577 Free PMC article.
-
Promising role of ANGPTL4 gene in diabetic wound healing.Int J Low Extrem Wounds. 2014 Mar;13(1):58-63. doi: 10.1177/1534734614520704. Int J Low Extrem Wounds. 2014. PMID: 24659626 Review.
-
Loss of keratinocyte focal adhesion kinase stimulates dermal proteolysis through upregulation of MMP9 in wound healing.Ann Surg. 2014 Dec;260(6):1138-46. doi: 10.1097/SLA.0000000000000219. Ann Surg. 2014. PMID: 25389925
-
Angiopoietin-like 4: a decade of research.Biosci Rep. 2012 Jun;32(3):211-9. doi: 10.1042/BSR20110102. Biosci Rep. 2012. PMID: 22458843 Review.
Cited by
-
Cytosolic and Transmembrane Protein Extraction Methods of Breast and Ovarian Cancer Cells: A Comparative Study.J Biomol Tech. 2018 Sep;29(3):71-78. doi: 10.7171/jbt.18-2903-002. J Biomol Tech. 2018. PMID: 30174558 Free PMC article.
-
Single-Cell Transcriptomics of Proliferative Phase Endometrium: Systems Analysis of Cell-Cell Communication Network Using CellChat.Front Cell Dev Biol. 2022 Jul 22;10:919731. doi: 10.3389/fcell.2022.919731. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35938159 Free PMC article.
-
Angiopoietin-like 4 Mediates Colonic Inflammation by Regulating Chemokine Transcript Stability via Tristetraprolin.Sci Rep. 2017 Mar 13;7:44351. doi: 10.1038/srep44351. Sci Rep. 2017. PMID: 28287161 Free PMC article.
-
The role of extracellular matrix in the pathophysiology of diabetic wounds.Matrix Biol Plus. 2020 Apr 22;6-7:100037. doi: 10.1016/j.mbplus.2020.100037. eCollection 2020 May. Matrix Biol Plus. 2020. PMID: 33543031 Free PMC article.
-
Angiopoietin-like 4 (Angptl4): A glucocorticoid-dependent gatekeeper of fatty acid flux during fasting.Adipocyte. 2012 Jul 1;1(3):182-187. doi: 10.4161/adip.20787. Adipocyte. 2012. PMID: 23700531 Free PMC article.
References
-
- Hynes R. O. (2002) Cell 110, 673–687 - PubMed
-
- Werner S., Grose R. (2003) Physiol. Rev. 83, 835–870 - PubMed
-
- Bornstein P., Sage E. H. (2002) Curr. Opin Cell Biol. 14, 608–616 - PubMed
-
- Bulcão C., Ferreira S. R., Giuffrida F. M., Ribeiro-Filho F. F. (2006) Curr. Diabetes Rev. 2, 19–28 - PubMed
-
- Caswell P. T., Norman J. C. (2006) Traffic 7, 14–21 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

