Nuclear factor I-C regulates TGF-{beta}-dependent hair follicle cycling
- PMID: 20729551
- PMCID: PMC2962510
- DOI: 10.1074/jbc.M110.120659
Nuclear factor I-C regulates TGF-{beta}-dependent hair follicle cycling
Abstract
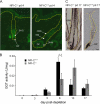
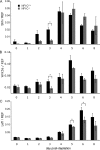
Skin appendages such as teeth and hair share several common signaling pathways. The nuclear factor I C (NFI-C) transcription factor has been implicated in tooth development, but a potential role in hair growth had not been assessed. In this study we found that NFI-C regulates the onset of the hair growth cycle. NFI-C(-/-) mice were delayed in the transition from the telogen to anagen phase of the hair follicle cycle after either experimental depilation or spontaneous hair loss. Lack of NFI-C resulted in delayed induction of the sonic hedgehog, Wnt5a, and Lef1 gene expression, which are key regulators of the hair follicle growth initiation. NFI-C(-/-) mice also showed elevated levels of transforming growth factor β1 (TGF-β1), an inhibitor of keratinocyte proliferation, and of the cell cycle inhibitor p21 at telogen. Reduced expression of Ki67, a marker of cell proliferation, was noted at the onset of anagen, indicating impaired activation of the hair progenitor cells. These findings implicate NFI-C in the repression of TGF-β1 signaling during telogen stage, resulting in the delay of progenitor cell proliferation and hair follicle regeneration in NFI-C-deficient mice. Taken together with prior observations, these findings also designate NFI-C as a regulator of adult progenitor cell proliferation and of postnatal tissue growth or regeneration.
Figures





Similar articles
-
Integrin β6-deficient mice show enhanced keratinocyte proliferation and retarded hair follicle regression after depilation.J Invest Dermatol. 2012 Mar;132(3 Pt 1):547-55. doi: 10.1038/jid.2011.381. Epub 2011 Nov 24. J Invest Dermatol. 2012. PMID: 22113470
-
Neural Stem Cells Restore Hair Growth Through Activation of the Hair Follicle Niche.Cell Transplant. 2016;25(8):1439-51. doi: 10.3727/096368916X691466. Epub 2016 Apr 22. Cell Transplant. 2016. PMID: 27110030
-
Overexpression of Wnt5a in mouse epidermis causes no psoriasis phenotype but an impairment of hair follicle anagen development.Exp Dermatol. 2014 Dec;23(12):926-8. doi: 10.1111/exd.12539. Epub 2014 Oct 10. Exp Dermatol. 2014. PMID: 25219536
-
STAT5 Activation in the Dermal Papilla Is Important for Hair Follicle Growth Phase Induction.J Invest Dermatol. 2016 Sep;136(9):1781-1791. doi: 10.1016/j.jid.2016.04.014. Epub 2016 Apr 27. J Invest Dermatol. 2016. PMID: 27131881 Review.
-
[Development of hair follicle stem cells related signal transduction in proliferation and differentiation].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010 Feb;24(2):161-4. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010. PMID: 20187446 Review. Chinese.
Cited by
-
Identification of common molecular signatures of SARS-CoV-2 infection and its influence on acute kidney injury and chronic kidney disease.Front Immunol. 2023 Mar 21;14:961642. doi: 10.3389/fimmu.2023.961642. eCollection 2023. Front Immunol. 2023. PMID: 37026010 Free PMC article.
-
The molecular anatomy of cashmere goat hair follicle during cytodifferentiation stage.BMC Genomics. 2024 Oct 15;25(1):961. doi: 10.1186/s12864-024-10820-2. BMC Genomics. 2024. PMID: 39407092 Free PMC article.
-
Integrated Analysis of Methylome and Transcriptome Changes Reveals the Underlying Regulatory Signatures Driving Curly Wool Transformation in Chinese Zhongwei Goats.Front Genet. 2020 Jan 8;10:1263. doi: 10.3389/fgene.2019.01263. eCollection 2019. Front Genet. 2020. PMID: 31969898 Free PMC article.
-
SNP Discovery from Transcriptome of Cashmere Goat Skin.Asian-Australas J Anim Sci. 2015 Sep;28(9):1235-43. doi: 10.5713/ajas.15.0172. Asian-Australas J Anim Sci. 2015. PMID: 26323515 Free PMC article.
-
SIRT7 activates quiescent hair follicle stem cells to ensure hair growth in mice.EMBO J. 2020 Sep 15;39(18):e104365. doi: 10.15252/embj.2019104365. Epub 2020 Jul 21. EMBO J. 2020. PMID: 32696520 Free PMC article.
References
-
- Roulet E., Bucher P., Schneider R., Wingender E., Dusserre Y., Werner T., Mermod N. (2000) J. Mol. Biol. 297, 833–848 - PubMed
-
- Gronostajski R. M. (2000) Gene 249, 31–45 - PubMed
-
- Gründer A., Ebel T. T., Mallo M., Schwarzkopf G., Shimizu T., Sippel A. E., Schrewe H. (2002) Mech. Dev. 112, 69–77 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

